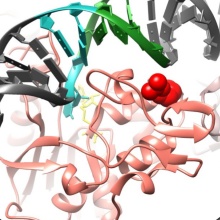
Up to 25% of patients with acute myeloid leukemia (AML) have mutations in an enzyme responsible for regulating DNA methylation. Researchers led by Prof. Albert Jeltsch of the University of Stuttgart are trying to better understand the biological effects of these mutations. If they are successful, this could lead to new approaches to treating such tumors. The project was funded by the Wilhelm Sander Foundation.
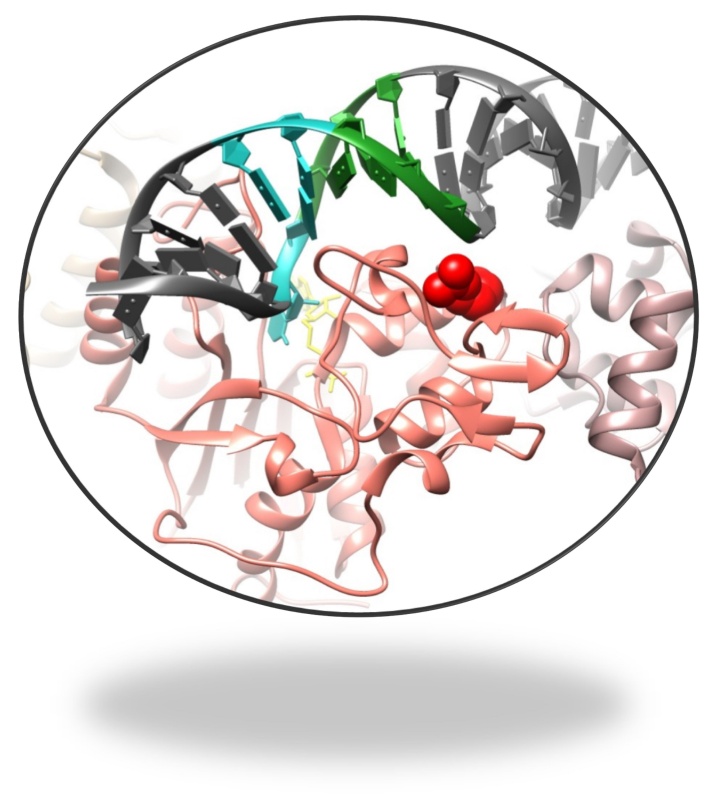
Leukemia is a malignant disease of the bone marrow and lymphatic system, which are responsible for producing red and white blood cells. In leukemia patients, the normal process of cell production is impaired. The cells divide in an uncontrolled and uninhibited manner. As a result, they do not mature and are unable to perform their actual function. A distinction is made between acute forms of leukemia, which occur rapidly and violently, and chronic forms, which develop slowly. Acute myeloid leukemia is the most common type of acute leukemia in adults. In patients with AML, the immature precursors of white blood cells (i.e. granulocytes or monocytes) degenerate.
Whether a certain gene in the genetic material (DNA) of a cell becomes active – or is “expressed” – is controlled by enzymes, including DNA methyltransferases, which modify specific sites of DNA by transferring a methyl group (CH₃). The methyl groups are inserted at sites on the DNA consisting of sequences of the bases cytosine and guanosine (CpG sequences), which play an important role in regulating gene expression.
Alterations in DNMT3A DNA methyltransferase are often found in AML tumors. Researchers led by Albert Jeltsch have studied how these mutations alter the properties of the enzyme. The two most common variants are R882H and R736H. Using a newly developed “deep enzymology technology” (Jeltsch et al., 2021), the research group showed that the R882H variant exhibits strongly altered methylation preferences compared with the non-mutated enzyme (wild type) (Emperle et al., 2019).
The resulting altered methylation is associated with a dysregulation of several genes closely associated with tumor progression. These observations provide detailed insight into the mechanisms by which the R882H cancer mutation affects the activity of cells.
Because all genes are duplicated in a cell, the unmodified enzyme (wild type) and the R882H mutation occur in parallel. It was therefore unclear why the effects of the mutant were so pronounced. Also this question was answered in the project funded by the Wilhelm Sander Foundation (Mack et al., 2022). The formation of enzyme complexes from wild-type and the R882H mutant occurs in a manner in which the altered properties of the R882H mutant dominate those of the wild-type enzyme.
How R736H, the second most common mutation of DNMT3A DNA methyltransferase, affects cancer progression was not known at the start of the project. Experimental studies have now shown that this mutant is stimulated under certain conditions (Kunert et al., 2022). As with R882H, this can alter the methylation of DNA in cancer cells, thereby modulating gene expression. Inhibition of the mutant DNMT3A enzymes R882H and R736H could therefore contribute to the treatment of such tumors.
The Wilhelm Sander foundation
The Wilhelm Sander Foundation supported the research project with almost EUR 240,000 over 24 months. The purpose of the foundation is to promote medical research, particularly in projects that contribute towards the fight against cancer. Since the foundation was established, over EUR 250 million has been paid out for research funding in Germany and Switzerland. This makes the Wilhelm Sander Foundation one of the most important private research foundations in Germany. It emerged from the estate of entrepreneur Wilhelm Sander, who passed away in 1973.
Expert Contact:
Prof. Dr. Albert Jeltsch, University of Stuttgart, Institute of Biochemistry and Technical Biochemistry, Department of Biochemistry, Tel. +49 711 685 64390, E-Mail
Publications:
Kunert S, Emperle M, Adam S, Dukatz M, Rajavelu A, Jeltsch A. The R736H cancer mutation in DNMT3A modulates the properties of the FF subunit interface. Biochimie (2022)
Mack A, Emperle M, Schnee P, Adam S, Pleiss J, Bashtrykov P, Jeltsch A. Preferential self-interaction of DNA methyltransferase DNMT3A subunits containing the R882H cancer mutation leads to dominant changes of flanking sequence preferences. J Mol Biol 434, 167482 (2022).
Jeltsch A, Adam S, Dukatz M, Emperle M, Bashtrykov P. Deep enzymology studies
on DNA methyltransferases reveal novel connections between flanking sequences
and enzyme activity. J Mol Biol 433, 167186 (2021).
Emperle M, Adam S, Kunert S, Dukatz M, Baude A, Plass C, Rathert P, Bashtrykov P,
Jeltsch A. Mutations of R882 change flanking sequence preferences of the DNA
methyltransferase DNMT3A and cellular methylation patterns. Nucleic Acids Res 47,
11355-11367 (2019).