Researchers all over the world are looking for new and affordable ways to create “clean” fuel. Although battery-powered cars have been on the roads for a long time now, converting aircraft or container ships to battery power for example is not possible, since the batteries required would be too large and too heavy. However, a synthetic fuel made from renewable materials being developed by the universities of Stuttgart and Bayreuth along with two partners from industry as part of the new “PlasmaFuel” research project may change this.
Dirty crude oil used by diesel engines in ships are responsible for a considerable proportion of the world’s greenhouse gas emissions, and the turbines of passenger and cargo planes are a major contributor to the high levels of CO2 emitted into the atmosphere. Synthetic fuels which do not use fossil raw materials would therefore go a long way towards achieving climate targets.
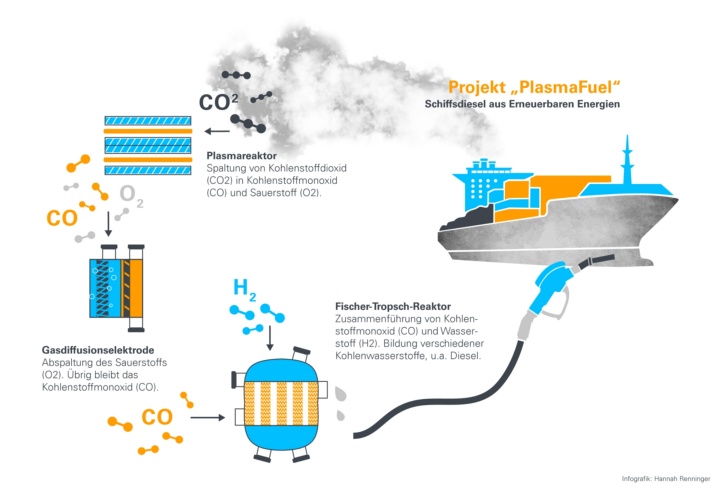
In order to develop a fuel of this kind, the researchers working on the PlasmaFuel project want to use carbon dioxide from the air or from industrial emissions, for example from cement works, surplus power from renewable energies as well as hydrogen from electrolysis. The CO2 is split into carbon monoxide (CO) and oxygen (O2) in a plasma reactor. In the last stage in the process, a so-called Fischer-Tropsch reactor synthesizes hydrocarbon chains which are made from carbon monoxide and hydrogen such as diesel or kerosene. The fuel produced can then be used directly in the ship’s engine or the aircraft in the same way as conventional petroleum products. The process can be operated efficiently by using intelligent control systems which take the supply of renewable energy into consideration as well as the different stages of synthesis.
Partner institutions throughout the process chain
In order to realize this approach, partner institutions from across Germany throughout the entire process chain have joined forces as part of the PlasmaFuel project. This brilliant idea comes from the medium-sized company MCT Transformatoren GmbH, which specializes in plasma processes as a way of cleaning the air, for example in large kitchens or recreation rooms. The Institute for Photovoltaics (IVP) at the University of Stuttgart with the Electrical Energy Storage Systems group uses this technology to split CO2, and is developing it further into the so-called Dielectric Barrier Discharge (DBD) plasma reactor.
Robust, cost-effective and scalable
In this plasma reactor, the carbon dioxide flows into a compartment in which a plasma forms between two electrode plates. The high energy of the electrons in the plasma splits the carbon dioxide molecules into carbon monoxide and oxygen. To make this possible, the IVP carries out research in particular into the extent to which the change in frequency of the alternating voltage influences the conversion to gas. The advantages of this process include the robust operation, the cheap raw materials, as well as excellent scalability for use in large industrial facilities.
The highly-reactive oxygen which is produced during this process must be extracted afterwards. The IVP has also developed a gas diffusion electrode (GDE), which enables it to “sort out” (separate) oxygen electrochemically and with a high degree of efficiency at low temperatures and low atmospheric pressure.
The actual fuel is formed from carbon monoxide using a so-called Fischer-Tropsch synthesis at the Department of Chemical Engineering (CVT) at the University of Bayreuth. The CVT investigates which impairments result from excess oxygen from the plasma process during synthesis and how this can be avoided. The group’s second aim is for as high a proportion as possible of the hydrocarbons created during the synthesis to have a suitable chain length. This is because hydrocarbons with a short chain are gaseous in form and are not suitable as fuel. If chains are too long however, waxes start to form which must be laboriously removed from certain types of fuels such as kerosene. The optimum chain length for the actual end product of the liquid hydrocarbons is somewhere in between.
Stabilizing the entire energy network
The energy required for the process comes from so-called surplus energy, which depending on the weather comes from solar or wind power. This means that the future “PlasmaFuel” fuel plant will be able to stabilize the entire energy network by running the processes all the time even when more energy is available than is needed. To ensure that the process runs smoothly, all facilities and processes must be accurately synchronized with one another in real time. This requires precise calibration – which Overspeed GmbH & Co. KG in northern Germany specializes in. The exchange of data is already subjected to rigorous testing for work carried out at a laboratory scale, which means that there will soon be nothing stopping the process from being applied on an industrial scale.
Expert Contact:
Prof. Peter Birke, University of Stuttgart, Institute for Photovoltaics, Electrical Energy Storage Systems working group, Tel. +49 711 685-67180, E-Mail