Scientists from the 2. Physics Institute at the University of Stuttgart and the Max Planck Institute for Medical Research were now able to take the next step towards synthetic cells: They introduced functional DNA-based cytoskeletons into cell-sized compartments and showed functionality. The results were recently published in Nature Chemistry.
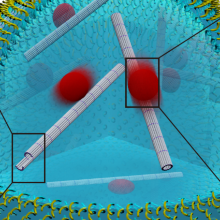
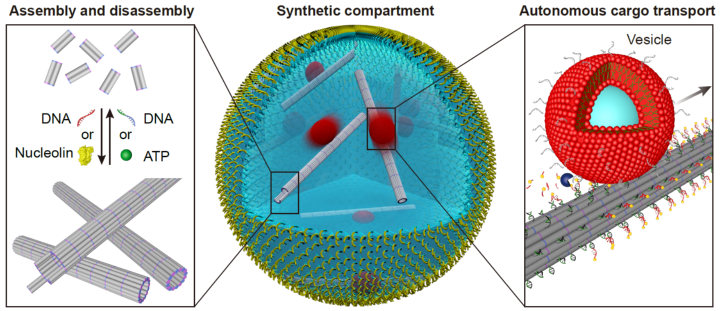
Building functional synthetic cells from the bottom-up is an ongoing effort of scientists around the globe. Their use in studying cellular mechanisms in a highly controlled and pre-defined setting creates great value for understanding nature as well as developing new therapeutic approaches. Scientists from the 2. Physics Institute at the University of Stuttgart and colleagues from the Max Planck Institute for Medical Research were now able to take the next step towards synthetic cells. They introduced functional DNA-based cytoskeletons into cell-sized compartments. Cytoskeletons are essential components of each cell that control their shape, internal organization and other vital functions such as transport of molecules between different parts of the cell. Upon incorporating the cytoskeletons into the synthetic droplets, the researchers also showed functionality such as transport of molecules or assembly and disassembly upon certain triggers. The results were recently published in Nature Chemistry.
Challenge to mimic cytoskeletal functions
The cytoskeleton is a crucial component of each cell and it is made up of various proteins. Beyond the basic function of giving the cell its shape, it is essential for many cellular processes such as cell division, intracellular transport of various molecules or motility in response to external signaling. Due to its importance in natural systems, being able to mimic its functionality also in an artificial setup is an important step towards building and designing a synthetic cell. However, it comes with many challenges due to the diverse requirements towards it, including stability as well as quick adaptability and reactivity to triggers.
Researchers in the field of synthetic biology have previously used DNA nanotechnology to recreate cellular components such as DNA-based mimics of ion channels or cell-cell linkers. For this, they take advantage of the fact that DNA can be programmed or engineered to self-assemble into a pre-planned shape by complementary base-pairing.
DNA filaments as synthetic cytoskeleton
“Synthetic DNA structures can enable highly specific and programmed tasks as well as versatile design possibilities beyond what is available from the biologically defined tools. Especially, the structural organization of the DNA structures may depart from their natural counterparts, even possibly outpacing the functionality scope of natural systems”, says Laura Na Liu, Professor at the 2. Physics Institute, University of Stuttgart.
Furthermore, Researchers such as Paul Rothemund, Elisa Franco or Rebecca Schulman, had already been successful in assembling DNA into micron-scale filaments, which constitute the basis of building a cytoskeleton. Since then, these filaments have been equipped with diverse functions, such as the assembly and disassembly upon external stimulation or inside a compartment. Scientists from the University of Stuttgart and the MPI for Medical Research have now taken the next step to building an artificial cell, by using the filaments as a synthetic cytoskeleton and giving them diverse functionality.
“It is exciting that we can also trigger the assembly of the DNA cytoskeleton with ATP – the same molecule cells use to power different mechanisms”, says Kerstin Göpfrich, Max Planck Research Group Leader at the MPI for Medical Research.
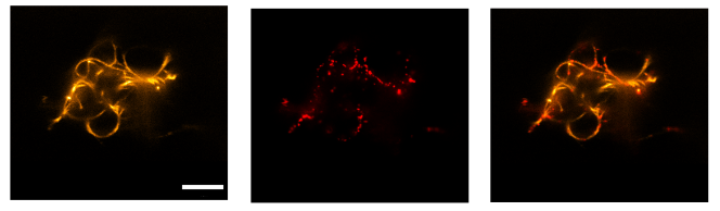
Speeding up the vesicle transport
Moreover, the team of scientists was able to induce the transport of vesicles along the filaments using the burnt-bridge mechanism introduced by Khalid Salaita. This mimics the vesicle transport along parts of the natural cytoskeleton in cells, called microtubuli. “In comparison to transport in living cells, transport along our DNA filaments is still slow. Speeding it up will be a challenge for the future”, says Kevin Jahnke, shared first author of the paper and postdoc in Kerstin Göpfrich’s group at the MPIMR. Pengfei Zhan, postdoc in the group led by Prof. Laura Na Liu in Stuttgart, adds: “It was also a challenge to fine-tune the energy landscapes of the DNA nanostructure’s assembly and disassembly capabilities of the filaments.” In the future, functionalizing the DNA filaments even more will be crucial to mimic natural cells even better. Thereby, researchers could create synthetic cells to study cellular mechanisms in greater detail or develop new therapeutic approaches.
Expert Contact:
Prof. Laura Na Liu, Universität Stuttgart, 2. Physikalisches Institut,
Tel. +49 711 685-65218, E-Mail