Research Topics Prof. Dr. Michael R. Buchmeiser
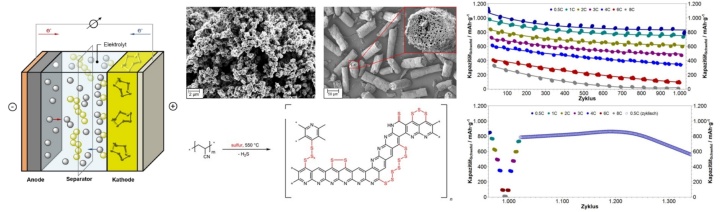
Sulfurated polymers such as sulfurated poly(acrylonitrile) (SPAN), poly(dicyclopentadiene) (S-polyDCPD), poly(norbornadiene) (S-polyNBDE), polystyrene (S-PS), poly(butadiene (S-polyBD) and poly(propylene) (S-PP) containing up to 69 wt.-% of chemically bound S are used as cathode materials for M-S batteries (M = Li, Mg). Different morphologies, including fibrous, monolithic and pellicular structures, are employed. The structure of the sulfurated polymers is fully elucidated und correlated with the electrochemical performance of metal-S batteries built therefrom. In particular, their discharge / charge chemistry and the role of the electrolyte during cycling are investigated. Investigations entail physico-chemical as well as electrochemical measurements including WAXS, ESCA, FT-IR, Raman, MALDI-TOF, XPS, SEM, EDX, electric impedance spectroscopy and cyclic voltammetry to name just a few. Based on that knowledge, Li-S, Na-S and Mg-S batteries stable for >1200 cycles displaying energy densities up to 2 mA.h/cm2 @ 0.5C have already been realized and new cathode/anode(electrolyte combinations are currently under Investigation. In addition, new electrolytes and optimized Si- and C-based anodes are developed to improve cycle stability, C-rate capability and discharge capacities of M-S cells. In summary, a comprehensive picture of the chemistry and electrochemistry of M-S batteries is to be created that ultimately allows for designing high-capacity devices with good cycling stability (>1500 cycles) and high energy density (>3.5 mA.h/cm2 @ 1C).
Selected Publications:
- J. Trück, P. Wang, E. Buch, J. Groos, S. Niesen, M. R. Buchmeiser, J. Electrochem. Soc. 2021, 169, 010505.
- P. Wang, M. R. Buchmeiser, Adv. Funct. Mater. 2019, 29, 1905248.
- P. Wang, J. Kappler, B. Sievert, J. Häcker, K. Müller, M. R. Buchmeiser, Electrochim. Acta. 2020, 361, 137024.
- P. Wang, K. Küster, U. Starke, C. Liang, R. Niewa, M. R. Buchmeiser, J. Power Sources 2021, 515, 230604.
- P. Wang, J. Trück, J. Häcker, A. Schlosser, L. Reinders, M. R. Buchmeiser, Energy Storage Mater. 2022, 49, 509.
- P. Wang, J. Trück, S. Niesen, J. Kappler, K. Küster, U. Starke, F. Ziegler, A. Hinntenach, M. R. Buchmeiser, Batteries & Supercaps 2020, 3, 1239.
- S. Niesen, J. Kappler, J. Trück, L. Veith, T. Weil, T. Soczka Guth, M. R. Buchmeiser, J. Electrochem. Soc. 2021, 168, 050510.
- S. Niesen, J. Trück, C. Seidl, K. Renger, M. R. Buchmeiser, J. Electrochem. Soc. 2021, 168, 110513.
- Q. Du, A. Fox, M. Benedikter, J. Kappler, K. Küster, T. Acartürk, U. Starke, M. R. Buchmeiser, ACS Appl. Energy Mater. 2022, 5, 7642.
- A. M. Fox, S. Niesen, Q. Du, N. Keim, D. Vrankovic, M. R. Buchmeiser, J. Electrochem. Soc. 2022, 169, 100507.
- A. M. Fox, D. Vrankovic, M. R. Buchmeiser, Appl. Mater. Interf. 2022, 14, 761.
- S. Niesen, A. M. Fox, S. Murugan, G. Richter, M. R. Buchmeiser, ACS Appl. Energy Mater. 2022, 5, 11386.
- S. Murugan, S. Niesen, K. Küster, U. Starke, J. Kappler, M. R. Buchmeiser, Batteries & Supercaps 2021, 4, 1636.
- M. Frey, R. K. Zenn, S. Warneke, K. Müller, A. Hintennach, R. E. Dinnebier, M. R. Buchmeiser, ACS Energy Lett. 2017, 2, 595.
- S. Warneke, M. Eusterholz, R. Zenn, A. Hintennach, R. E. Dinnebier, M. R. Buchmeiser, J. Electrochem. Soc. 2018, 165, A6017.
- S. Warneke, M. Eusterholz, R. K. Zenn, A. Hintennach, R. E. Dinnebier, M. R. Buchmeiser, J. Electrochem. Soc. 2017, 165, A6017.
- S. Warneke, A. Hintennach, M. R. Buchmeiser, J. Electrochem. Soc. 2018, 165, A2093.
- S. Warneke, R. K. Zenn, T. Lebherz, K. Müller, A. Hintennach, U. Starke, R. E. Dinnebier, M. R. Buchmeiser, Adv. Sust. Syst. 2018, 2, 1700144.
- M. Frey, M. R. Buchmeiser, A. Hintennach, Kathodenmaterial und Verfahren zu dessen Herstellung, Daimler AG, Germany, DE 102014012468.1.
- T. Lebherz, M. Frey, A. Hintennach, M. R. Buchmeiser, RSC Adv. 2018, 9, 7181.
- T. Lebherz, D. L. Weldin, A. Hintennach, M. R. Buchmeiser, Macromol. Chem. Phys. 2020, 221, 1900436.
- J. Fanous, M. Wegner, J. Grimminger, A. Andresen, M. R. Buchmeiser, Chem. Mater. 2011, 23, 5024.
- J. Fanous, M. Wegner, J. Grimminger, M. Rolff, M. B. M. Spera, M. Tenzer, M. R. Buchmeiser, J. Mater. Chem. 2012, 22, 23240.
- J. Fanous, M. Wegner, M. B. M. Spera, M. R. Buchmeiser, J. Electrochem. Soc. 2013, 160, A1169.
- M. R. Buchmeiser, Q. Du, Cathode materials for lithium-sulfur batteries, University of Stuttgart, patents pending (2022).
- Q. Du, M. Benedikter, K. Küster, T. Acartürk, U. Starke, J.-L. Hoslauer, T. Schleid, M. R. Buchmeiser, Batt. & Supercaps 2022, 5, e202200277.
- J. Kappler, S. Klostermann, P. Lange, M. Vocht, M. Dyballa, L. Veith, T. Schleid, T. Weil, J. V. Kästner, M. R. Buchmeiser, Batteries & Supercaps 2023, 6, e202200522.
- J. Kappler, G. Tonbul, R. Schoch, S. Murugan, M. Nowakowski, P. Lange, S. Klostermann, M. Bauer, T. Schleid, J. Kästner, M. R. Buchmeiser, J. Electrochem. Soc. 2023, 170, 010526.
- Q. Du, A. Fox, L. Reinders, K. Küster, T. Acartürk, U. Starke, M. R. Buchmeiser, ACS Appl. Polym. Mater. 2023, 5, 5238-5245.
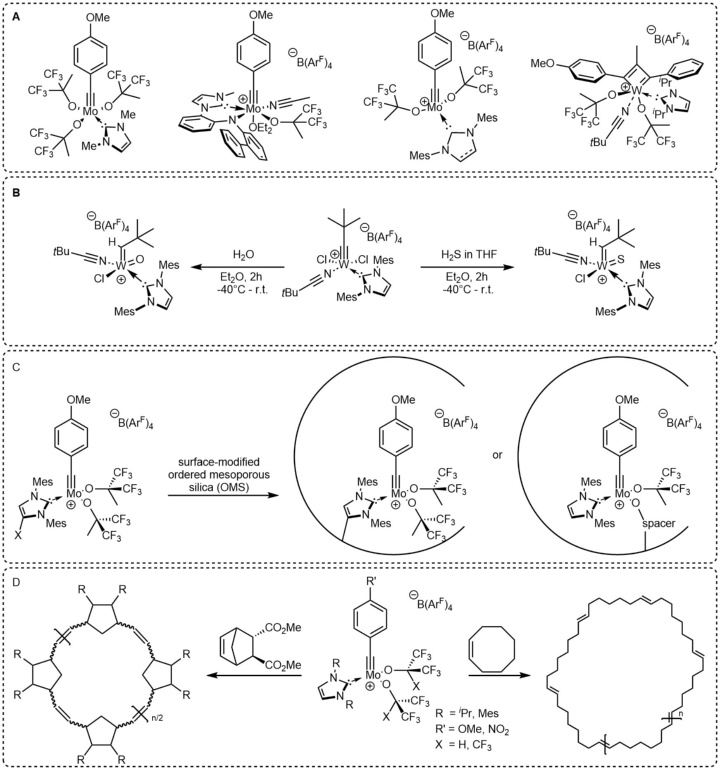
Recently, our group synthesized molybdenum and tungsten alkylidyne N-heterocyclic carbene (NHC) complexes for alkyne metathesis. Based on NMR experiments, a cationic species bearing strong σ-donor carbenes was identified as active catalyst.[1, 2] Accordingly, various cationic molybdenum and tungsten alkylidyne NHC complexes were isolated that showed high reactivity in alkyne metathesis reactions.[3-5] Especially a cationic molybdenum alkylidyne complex bearing the 1,3-dimesitylimidazol-2-ylidene moiety showed outstanding productivity and compatibility with various functional groups.[3]
The corresponding cationic tungsten alkylidyne NHC complexes possess lower activity in alkyne metathesis, yet allow for the isolation of cationic tungstacyclobutadiene NHC complexes, which represent an intermediate in the metathesis cycle.[4] Most important, the reaction of cationic tungsten alkylidyne NHC complexes with one equivalent of water offers access to a variety of cationic tungsten oxo alkylidene NHC complexes for olefin metathesis reactions. This direct synthetic route allows for the synthesis of novel alkylidene complexes, e.g. with the 1,3-dimesityl-4,5-dichloroimidazol-2-ylidene ligand.[6] Furthermore, the analogous reaction with H2S leads to tungsten sulfido alkylidene NHC complexes.[7]
To transfer the reactivity of these complexes into the pores of ordered mesoporous silica materials, different immobilization strategies are currently developed. Since the direct immobilization of alkylidyne complexes on silica surfaces is known to results in a strongly decreased productivity,[8] different surface modifications of the pores are currently tested to circumvent this issue. This work is directed towards the CRC1333, a collaborative research center dedicated to gaining knowledge on catalysis in confined geometries.
Most recently, group VI alkylidyne complexes were shown to promote olefin metathesis reactions, such as ring-closing metathesis[9] and ring-expansion metathesis polymerization (REMP).[10-12] The latter is of interest due to the lack of end groups in the resulting polymer, resulting in unique properties. We successfully use cationic molybdenum alkylidene NHC complexes in the stereoselective REMP of cyclic olefins and identify critical reaction parameters that influence both, molecular weight and molecular weight distribution.
Selected Publications:
- I. Elser, J. Groos, P. M. Hauser, M. Koy, M. van der Ende, D. Wang, W. Frey, K. Wurst, J. Meisner, F. Ziegler, J. Kästner, M. R. Buchmeiser, Organometallics 2019, 38, 4133.
- M. Koy, I. Elser, J. Meisner, W. Frey, K. Wurst, J. Kästner, M. R. Buchmeiser, Chem. Eur. J. 2017, 23, 15484.
- J. Groos, P. M. Hauser, M. Koy, W. Frey, M. R. Buchmeiser, Organometallics 2021, 40, 1178.
- P. M. Hauser, M. van der Ende, J. Groos, W. Frey, D. Wang, M. R. Buchmeiser, Eur. J. Inorg. Chem. 2020, 3070.
- J. Groos, M. Koy, J. Musso, M. Neuwirt, T. Pham, P. M. Hauser, W. Frey, M. R. Buchmeiser, Organometallics 2022; 241, 1167.
- P. M. Hauser, J. V. Musso, W. Frey, M. R. Buchmeiser, Organometallics 2021, 40, 927.
- P. M. Hauser, K. Gugeler, W. Frey, J. Kästner, M. R. Buchmeiser, Organometallics 2021, 40, 4026.
- D. P. Estes, C. Bittner, O. Arias, M. Casey, A. Fedorov, M. Tamm, C. Coperet, Angew. Chem. Int. Ed. 2016, 55, 13960.
- S. Chuprun, C. M. Acosta, L. Mathivathanan, K. V. Bukhryakov, Organometallics 2020, 39, 3453.
- S. A. Gonsales, T. Kubo, M. K. Flint, K. A. Abboud, B. S. Sumerlin, A. S. Veige, J. Am. Chem. Soc. 2016, 138, 4996.
- S. S. Nadif, T. Kubo, S. A. Gonsales, S. VenkatRamani, I. Ghiviriga, B. S. Sumerlin, A. S. Veige, J. Am. Chem. Soc. 2016, 138, 6408.
- Z. Miao, S. A. Gonsales, C. Ehm, F. Mentink-Vigier, C. R. Bowers, B. S. Sumerlin, A. S. Veige, Nat. Chem. 2021, 13, 792.
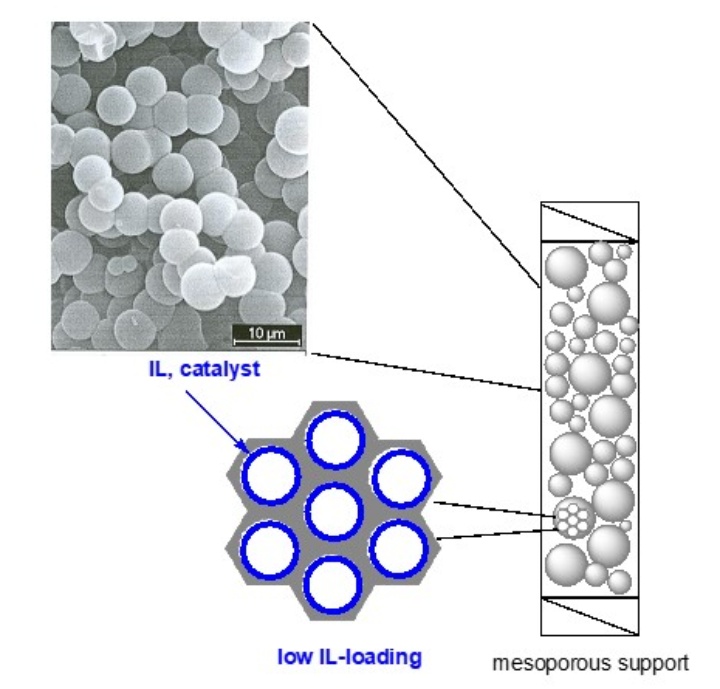
We already elaborated a successful SILP-based approach to olefin metathesis under supported biphasic conditions and a SILP-based approach to continuous heterogeneous biphasic enzyme catalysis. Currently, SILP technology is used to create a liquid confinement in the nanometer range by supporting nm-thick films inside the pores of mesoporous materials. The substrates are dissolved in a second organic phase that is immiscible with the IL; this way, reactions can be run under continuous biphasic conditions. In such a setup, an organometallic catalyst dissolved in the IL phase experiences a steric confinement generated by the two phase-boundaries, i.e. the solid-liquid and the liquid-liquid phase interface. This allows for tuning transition states and thus for running reactions (e. g. hydrosilylation, hydroboration, hydroamination reactions) with high stereospecifity.
Selected Publications:
- B. Autenrieth, W. Frey, M. R. Buchmeiser, Chem. Eur. J. 2012, 18, 14069.
- B. Sandig, M. R. Buchmeiser, ChemSusChem 2016, 9, 2917.
- B. Sandig, L. Michalek, S. Vlahovic, M. Antonovici, B. Hauer, M. R. Buchmeiser, Chem. Eur. J. 2015, 21, 15835.
- B. Sandig, M. R. Buchmeiser, Poröser monolithischer Hybridreaktor, ein Verfahren zu dessen Herstellung und seine Verwendung, University of Stuttgart, DE 102015113522.1.
- C. Lee, B. Sandig, M. R. Buchmeiser, M. Haumann, Catal. Sci. Technol. 2018, 8, 2460.
- M. R. Buchmeiser, ChemCatChem 2021, 13, 785.
- H. Acikalin, P. K. R. Panyam, A. Wasif Shaikh, D. Wang, S. R. Kousik, P. Atanasova, M. R. Buchmeiser, Macromol. Chem. Phys. 2022, 224, 2200234.
- P. K. R. Panyam, M. R. Buchmeiser, Faraday Disc. 2023, 244, 39-50.
- T. Beweries, M. R. Buchmeiser, N. R. Champness, M. Costas, A. Duhme-Klair, J. Echeverría, O. Eisenstein, C. T. J. Ferguson, J. C. Goodall, R. Gramage-Doria, M. Gyton, R. Ham, S. Herres-Pawlis, C. L. Johnson, P. Kennepohl, B. Lewandowski, P. R. Linnebank, S. A. Macgregor, K. T. Mahmudov, E. Meeus, M. Navarro, P. Ntola, T. N. Parac-Vogt, R. N. Perutz, A. Poater, D. C. Powers, S. Pullen, P. R. Raithby, J. N. H. Reek, T. R. Ward, A. S. Weller, H. Wennemers, Faraday Disc. 2023, 244, 96-118.
- T. Beweries, M. R. Buchmeiser, F. E. Bugden, N. R. Champness, B. Chanbasha, M. Costas, J. Echeverria, O. Eisenstein, C. Ferguson, J. C. Goodall, R. Gramage-Doria, M. Greenhalgh, M. Gyton, R. Ham, P. Kennepohl, B. Lewandowski, W.-C. Liu, S. A. Macgregor, K. T. Mahmudov, E. Meeus, J. Morris, P. Ntola, T. N. Parac-Vogt, R. N. Perutz, A. Poater, D. Powers, P. R. Raithby, J. N. H. Reek, I. Riddell, T. R. Ward, A. S. Weller, H. Wennemers, Faraday, Disc., 2023, 244, 434-454.
Carbon fibers are made of anisotropic carbon with at least 92 wt.-% and up to 100 wt.-% carbon. Carbon fibers have high tensile strengths of up to 7 GPa with very good creep resistance, low densities (ρ = 1.75-2.20 g/cm3) and high moduli of up to 950 GPa. They lack resistance to harsh oxidizing agents as hot air and flames, but they are resistant to all other chemical species. The unparalleled mechanical properties make carbon fibers attractive for use in composites, which are commonly referred to as carbon fiber reinforced plastics (CFRP), where carbon fibers are used in the form of woven textiles, continuous fibers or chopped fibers. The CFRPs can be produced through filament winding, tape winding, pultrusion, compression molding, vacuum bagging, liquid molding, and injection molding. For the automotive industry, CFRPs allow for a significant reduction in weight. More recently, carbon fibers moved into the center of interest as a substitute for steel in reinforced concrete. Because of their much higher resistance towards oxidation, carbon fibers in reinforced concrete enable thinner concrete structures, therefore reducing the needed amount of the increasingly scarce sand.
The most important precursor (>90 % market share) for carbon fiber production is poly(acrylonitrile) (PAN), the only other commercially available precursor being pitch. PAN-based carbon fibers inhibit high tensile strength (HT) and intermediate modulus (IMS), suitable for high strength CFRP applications, while pitch-based carbon fibers inhibit a lower tensile strength and a high modulus (HM) or “ultra” high modulus (UHM) for CFRP parts with high stiffness requirements.
However, in order to reduce energy consumption, costs and the environmental impact of carbon fiber production, renewable and energy-efficient precursors like lignin, cellulose or poly(ethylene) have moved into the center of interest. Current projects focus on all these precursors as well as on more cost-effective and environmentally friendly processing techniques. The ultimate goal is to dispose over an armor of better and more efficient processes for carbon fiber production, “green precursors” as well as carbon fibers with improved properties. Research is carried out at the Institute of Polymer Chemistry and the High-Performance Fiber Center (HPFC) at the DITF, where pilot lines for carbon fiber production enable the processing of common and novel precursors and the production of carbon fibers on a kilogram scale.
Selected Publications:
- E. Frank, M. R. Buchmeiser "Fiber, films, resins and plastics" in Encyclopedia in Polymeric Nanomaterials (S. Kobayashi, K. Müllen, Eds.), Springer, 2015, 1, 306-310, ISBN: 978-642-29647-5.
- E. Frank, D. Ingildeev, L. M. Steudle, J. M. Spörl, M. R. Buchmeiser, Angew. Chem., 2014, 126, 5364-5403; Angew. Chem. Int. Ed., 2014, 53, 5262-5298.
- E. Frank, F. Hermanutz, M. R. Buchmeiser, Macromol. Mater. Eng., 2012, 297, 493-501.
- E. Frank, E. Giebel, M. R. Buchmeiser, Techn. Text., 2, 2015, E53-55; Chem. Fibers, Int., 2015, 4, 216-218.
- E. Frank, D. Ingildeev, M. R. Buchmeiser, “High-Performance Poly(acrylonitrile) (PAN)-Based Carbon Fibers” in Structure and Properties of High-Performance Fibers, (G. Bhat, Ed.), 1stEd. , Woodhead Publishing Ltd., 2016, 187, 7-30, ISBN 978-0-08-100550-7.
- J. M. Spörl, A. Ota, R. Beyer, T. Lehr, A. Müller, F. Hermanutz, M. R. Buchmeiser, J. Polym. Sci. A: Polym. Chem., 2014, 52, 1322-1333.
- J. M. Spörl, A. Ota, S. Sun, K. Massonne, F. Hermanutz, M. R. Buchmeiser, Mater. Today Commun., 2016, 7, 1-10.
- S. Son, K. Massonne, F. Hermanutz, J. Spoerl, M. R. Buchmeiser, R. Beyer (BASF E), PCT Int. Appl. WO 2015173243 A1.
- L. Steudle, E. Frank, A. Ota, U. Hageroth, S. Henzler, W. Schuler, R. Neupert, M. R. Buchmeiser, Macromol. Mater. Eng., 2017, 302, 1600441.
- M. Clauss, E. Frank, M. R. Buchmeiser (DITF Denkendorf), WO2017089585A1.
- E. Frank, E. Muks, M. R. Buchmeiser (DITF Denkendorf), DE102015106348A1.
- J. W. Krumpfer, E. Giebel, A. Müller, L. Ackermann, C. Nardi-Tironi, J. Unold, M. Klapper, M. R. Buchmeiser, K. Müllen, Chem. Mater., 2017, 29, 780-788.
- M. Speiser, S. Henzler, U. Hageroth, A. Renfftlen, A. Müller D. Schawaller, B. Sandig, M. R. Buchmeiser, Carbon, 2013, 63, 554-561.
- M. R. Buchmeiser, J. Unold, K. Schneider, E. B. Anderson, F. Hermanutz, E. Frank, A. Müller, S. Zinn, J. Mater. Chem. A, 2013, 1, 13154-13163.
- S. König, M. M. Clauss, E. Giebel, M. R. Buchmeiser, Polym. Chem., 2019, 10, 469-4476.
- E. Frank, E. Giebel, M. R. Buchmeiser, S. König, (DITF Denkendorf), DE102017127629A1.
- M. R. Buchmeiser, E. Muks, E. Frank, U. Hageroth, S. Henzler, R. Schowner, J. Spörl, A. Ota, R. Beyer, Carbon, 2019, 144, 659-665.
- M. R. Buchmeiser, E. Muks, E. Frank, U. Hageroth, S. Henzler, R. Schowner, J. Spörl, A. Ota, R. Beyer, Structure Evolution in All-Aromatic, Poly(p-Phenylene-Vinylene)-Derived Carbon Fibers, Carbon, 2019, 144, 659-665.
- E. Frank, E. Muks, A. Ota, T. Herrmann, M. Hunger, M. R. Buchmeiser, Structure Evolution in Polyethylene-Derived Carbon Fibres Using a Combined Electron Beam-Stabilization-Sulphurization Approach, Macromol. Mater. Eng. 2021, 306, 2100280.
- M. P. Vocht, A. Ota, E. Frank, F. Hermanutz, M. R. Buchmeiser, ACS Ind. Eng. Chem. Res., 2022, 61, 5191−5201.
- M. L. Chacón-Patiño, A. Neumann, C. P. Rüger, P. G. Bomben, L. Friederici, R. Zimmermann, E. Frank, P. Kreis, M. R. Buchmeiser, M. R. Gray Chemistry and Properties of Carbon Fiber Feedstocks from Bitumen Asphaltenes, Energy & Fuels 2023, 37, 5341-5360.
- P. Kreis, E. Frank, B. Clauß, V. Bauch, H. Stolpmann, L. Kuske, T. Schneck, S. König, M. R. Buchmeiser, Macromol. Mater. Eng. 2023, in press.
Ceramic matrix composites (CMCs) are an interesting material class, combining high-temperature stability and low densities of monolithic ceramics and a non-brittle fracture behavior, induced by ceramic fibers. They are used in flight turbines, for charge carriers for high temperature treatment or in burner nozzles. Oxide ceramic fibers determine the properties of CMCs and therefore have to meet special requirements such as high strength, long-term high-temperature stability as well as excellent resistance against oxidation, corrosion and creep. Particularly under mechanical stress and at high temperatures exceeding 1100°C, ceramic fibers tend to creep and brittleness increases due to grain growth, which can ultimately lead to failure of the entire device. The optimization of the ceramic fiber properties with regard to high creep resistance while maintaining strength and associated long-term high temperature resistance still represents a major topic in ongoing research in the field of ceramic fibers.
Research at the DITF Denkendorf focuses on the development of continuous oxide ceramic fibers of various compositions. The complete production process has been studied intensively and comprises the design of spinning dopes, the development of the dry spinning process as well as the thermal treatment including pyrolysis, calcination and sintering processes. Spinning dopes are prepared using a solution process, enabling a wide range of phase compositions in the ceramic fibers, and are characterized using rheology. Green fibers are gained via dry spinning on laboratory and pilot scale. Calcination and sintering of the green fibers is carried out in continuous processes and the resulting fibers are characterized using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and X-ray diffraction (XRD) as well as mechanical tests.
Alumina and mullite fibers have achieved a high level of development in the past years. Currently, the transfer of the technology into industrial scale is under progress with Saint-Gobain (F) as an industrial partner. Current research projects focus on the improvement of creep resistance, the reduction of grain growth in long time applications and the improvement of the textile processability of oxide ceramic fibers. For this purpose, the chemical composition of the oxide ceramic fibers is further varied in order to optimize the structures and the mechanical properties.
The microstructural optimization of alumina and mullite fibers by the incorporation of zirconia results in zirconia-toughened alumina (ZTA) and mullite (ZTM) fibers with a substantially inhibited grain growth at high temperatures. ZTM fibers show improved textile processability and increased tensile strength in comparison to mullite fibers. Current research focuses on the optimization of ZTA and ZTM fibers and their characterization at high temperatures. Further optimizations of alumina fibers using yttrium-aluminum-garnet (YAG), an oxide with exceptionally high creep resistance are ongoing; the same accounts for the doping of alumina fibers with different oxides to improve their creep resistance.
In addition to fiber development, the DITF also conducts experiments on the weaving of ceramic fibers into fabrics. In cooperation with industrial partners, CMC test plates and demonstrators are subsequently produced from the fabrics. Thus, it could be proven that the DITF fibers, branded “OxCeFi fibers”, are at least equivalent to the best commercial oxide ceramic fibers.
Selected Publications:
- D. Schawaller, B. Clauß, M. R. Buchmeiser, Macromol. Mater. Eng., 297, 2012, 502-522.
- S. Pfeifer, M. Bischoff, R. Niewa, B. Clauß, M. R. Buchmeiser, J. Eur. Ceram. Soc., 34, 2014, 1321-1328.
- Müller, T. Schleid, N. Müschenborn, A. Tautzenberger, A. Ignatius, B. Clauß, M. R. Buchmeiser, J. Eur. Ceram. Soc. 2014, 34, 3993.
- S. Pfeifer, P. Demirci, R. Duran, H. Stolpmann, A. Renfftlen, S. Nemrava, R. Niewa, B. Clauß, M. R. Buchmeiser, J. Eur. Ceram. Soc., 36, 2016, 725-731.
- Reinders, S. Pfeifer, S. Kröner, H. Stolpmann, A. Renfftlen, L. C. Greiler, B. Clauß, M. R. Buchmeiser, J. Eur. Ceram. Soc. 2021, 41, 3570.
- Clauß, S. Pfeifer, L. Reinders, M. R. Buchmeiser, Nachr. Chem. 2021, 69, 42.
Current Thesis Topics
- Polymeric cathode materials for Li-sulfur batteries
- Synthesis of cationic molybdenum / tungsten alkylidyne complexes for alkyne metathesis reactions under continuous biphasic conditions
- Alkyne metathesis reactions in confined geometries
- Ring expansion metathesis polymerization (REMP) for the synthesis of cyclic polymers
- Stereoselective olefin metathesis with chiral Mo- and W-alkylidene NHC complexes
- Stereoselective alkyne hydrosilylation, hydroamination, and hydroboration
- Polymeric cathode materials for Li-sulfur batteries
- Synthesis of cationic molybdenum and tungsten alkylidyne complexes for olefin metathesis reactions under continuous biphasic conditions
- Alkyne metathesis reactions in confined geometries
- Syn/anti interconversion of Mo- and W-alkylidene NHC complexes
- Stereoselective olefin metathesis with chiral Mo- and W-alkylidene NHC complexes
- Macrocyclization based on alkyne metathesis with cationic Mo- and W-alkylidine-NHC complexes
- Stereoselective alkyne hydrosilylation, hydroamination, and hydroboration
- Selective ethylene tri-/tetramerization with Cr-NHC complexes
- Artificial DNA: precision polymers
- Alkyne metathesis reactions in confined geometries
- Synthesis of cationic molybdenum and tungsten alkylidene complexes for metathesis reactions under continuous biphasic conditions
- Stereoselective catalysis in chiral confinement
- Macrocyclization based on alkyne metathesis with cationic Mo- and W-alkylidine-NHC complexes
- Stereoselective olefin metathesis with chiral Mo- and W-alkylidene NHC complexes
- Stereoselective alkyne hydrosilylation, hydroamination, and hydroboration
- Selective ethylene tri-/tetramerization with Cr-NHC complexes
- Einfluss der Zellstoffqualität auf die Strukturbildung bei der Carbonisierung von Cellulosepräkursoren (MA)
- Charakterisierung und Optimierung von Faserverbundmaterialien auf Basis reiner Cellulose für den Einsatz als nachhaltiges Verpackungsmaterial (BA, MA)
- Einsatz von Zellstoffen aus Einjahrespflanzen für die Verarbeitung mittels HighPerCell-Verfahren (BA, MA)
- Auswahl von Farbstoffen und Optimierung der Färbevefahren für HighPerCell-Filamenttextilien (MA)
- Biobasierter Flammschutz für neue Cellulosefilamentgarne (MA)
- Einfluss der Zellstoffzusammensetzung auf die Faserstrukturbildung beim Airgap Spinnen (MA)