Recent Publications
by Yannick Stöckl*, Katrin Gugeler, Celine M. Holzwarth, Wolfgang Frey, Sascha Wegner, Birgit Claasen, Anna Zens, Dietrich Gudat, Christian P. Sindlinger, Johannes Kästner*, and Sabine Laschat
Organometallics 2024, 43, 3, 330–340.
Enantioenriched boron chelates show promising synthetic and luminescent properties; however, the challenging synthesis makes these compounds scarce. In our earlier work, we established a chirality transfer from boron O,N-chelates toward enantioenriched C,N-chelates. This methodology proved to be quite robust, in terms of yields and selectivity. However, unexpected steric effects on stereocontrol prompted a deeper investigation of the chirality transfer. In order to gain a holistic understanding of this process, we studied the structure of the O,N- and C,N-chelates as well as the stability of the dative B–N bonds. Furthermore, the proposed ate-complex as a reaction intermediate could be characterized using heteronuclear (2D) NMR spectroscopy. For this ate-complex, a tridentate O,N,N-chelate effect of the borate anion with the Li-cation was observed. Additional experiments indicated that the borate formation governs the stereoselectivity of chirality transfer. For a successful chirality transfer, an unprecedented SN2-type breaking of the dative B–N bond with an organometallic nucleophile was identified by DFT calculations as the most likely reaction path. For other cases, decreased or inverse enantioselectivity was rationalized by a solvent-assisted pathway.
For further information please contact:
Prof. Sabine Laschat
Institute of Organic Chemistry
University of Stuttgart
Isomerization of terpenes is achieved by Brønsted and Lewis acids, but the resulting products are mixtures difficult to separate. We have now succeeded in developing a variant of the enzyme squalene-hopene cyclases that catalyzes this difficult isomerization with an unprecedented precision. We show how the fine-tuned control of the carbocation in the enzyme can increase the (+)-borneol selectivity from 1% to >90% (>99% de). In collaboration with the group of Sílvia Osuna, University of Girona, our combined experimental and computational data suggest that the orientation of key aromatic residues leads to a restructuring of the water network that facilitates the selective termination of the secondary isobornyl cation. This work extends our mechanistic understanding of carbocation rearrangements and sets the stage for a target-oriented skeletal reorganization of widely used terpenes.
by Julian Ludwig, Christian Curado-Carballada, Stephan C. Hammer, Andreas Schneider, Svenja Diether, Nico Kress, Sergi Ruiz-Barragán, Sílvia Osuna* and Bernhard Hauer*
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by Jan S. Florenski, Noah Schellander and Prof. Dr.-Ing. Elias Klemm
ChemCatChem 2024, e202301
For further information please contact
Prof. Elias Klemm
Institute of Technical Chemistry
University of Stuttgart
by S. Gergel, J. Soler, A. Klein, K. H. Schülke, B. Hauer, M. Garcia-Borràs and S. Hammer.
The direct regioselective oxidation of internal alkenes to ketones poses an important synthetic challenge. Now, directed evolution of a cytochrome P450 enzyme affords a ketone synthase that can efficiently oxidize internal arylalkenes directly to ketones with high chemo- and regioselectivity.
Nature Catalysis 2023
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by L. W. Zimmermann, R. Aghaei Hakkak, M. Ranjbar, Th. Schleid: "Crystal Structures and Thermal Analyses of Three New High-Energy Hydrazinium Hydro-closo-Borates"
Int. J. Hydrog. Energy 2024, 49, 1469–1477.
For further information please contact
Prof. Thomas Schleid
Institute of Inorganic Chemistry
University of Stuttgart
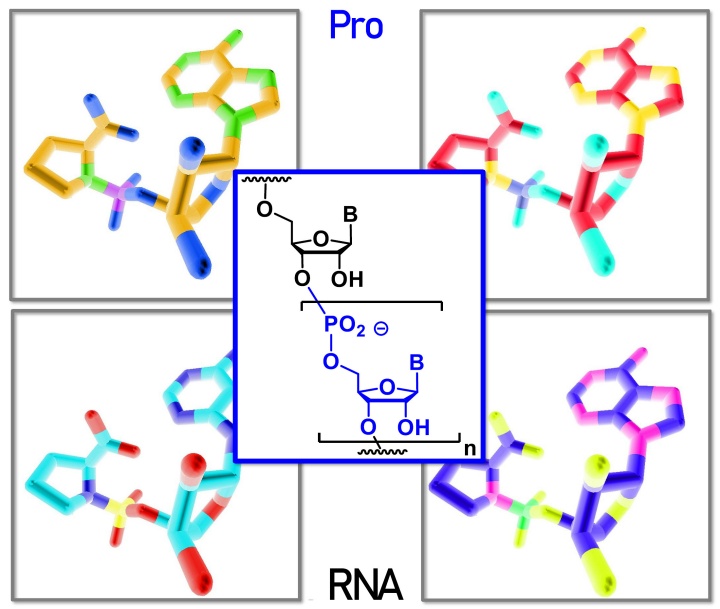
The paper "Prolinyl Nucleotides Drive Enzyme-Free Genetic Copying of RNA" from Prof. Richert was nominated for the cover of Angewandte (vide infra). It shows that long reads of mixed sequence RNA can be achieved with an amino acid as leaving group.
Angew. Chem. Int. Ed.2023, e202307591
For further information please contact:
Prof. Clemens Richert
Institute of Organic Chemistry
University of Stuttgart
by Shubhadeep Chandra, Dr. Arijit Singha Hazari, Dr. Qian Song, David Hunger, Dr. Nicolás I. Neuman, Prof. Dr. Joris van Slageren, Prof. Dr. Elias Klemm, Prof. Dr. Biprajit Sarkar.
For further information please contact:
Prof. Biprajit Sarkar
Institute of Inorganic Chemistry
University of Stuttgart
by Jona T. Schelle, William Lepottevin and Bernhard Hauer
Oxyfunctionalization reactions are of great interest in organic chemistry and industry. Rieske non-heme iron oxygenases, such as cumene dioxygenase, have shown significant potential for these reactions. However, scaling up oxygenase-catalyzed reactions can be challenging due to their reliance on molecular oxygen. In this study, we optimized the reaction set-up and oxygen uptake for cumene dioxygenase-catalyzed biotransformations and successfully scaled up the reaction volume from 1 mL to 200 mL. Our results demonstrate the applicability of our set-up for product isolation and characterization.
Chemie Ingenieur Technik 95,5: 607 – 611 (2023)
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by Benjamin Aberle, Daniel Kowalczyk, Simon Massini, Alexander-N. Egler-Kemmerer, Sebastian Gergel, Stephan Hammer, Bernhard Hauer
Terpenes are natural compounds with diverse applications, but the available carbon scaffolds are limited by their biosynthesis from five carbon precursors. To gain access to non-natural terpenoids, we identified and engineered methyltransferases for late-stage C-methylation of unactivated alkenes. The engineering resulted in a 55-fold improvement of conversion of (E,E)-farnesol with > 99% selectivity. In total, five non-natural terpenoids were produced and isolated using this biocatalytic method. This opens new avenues for the modification of the carbon scaffold of terpenes.
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by Andreas Schneider; Christian Curado; Thomas B. Lystbaek; Sílvia Osuna; Bernhard Hauer
Angewandte Chemie: DOI number 10.1002/anie.202301607
The synthetic power of terpene cyclases is of broad academic as well as industrial interest as it can cut down synthetic routes to complex cyclic terpenes to essentially one step. However, these enzymes usually lack catalytic turnovers and stability under the new-to-nature conditions. Teaming up with the BioCompLab, Girona of Silvia Osuna, we showcase the synergy of tailoring the active site and entrance tunnel of the squalene-hopene cyclase for the stereocontrolled cationic cyclization of E,E-homofarnesol to (–)-ambroxide with >100.000 total turnovers.
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by Kristina Schell, Heng Li, Lukas Lauterbach, Kizerbo A. Taizoumbe, Jeroen S. Dickschat and Bernhard Hauer
Enzyme active site confinement is important for efficient chemical reactions and catalysis. However, a strongly confined active site limits the range of substrates that can be used. In this study we developed a structure-guided strategy to create alternative confinement in the squalene-hopene cyclase enzyme, which aims to pre-organize geranyl acetone analogs with deviant isoprene patterns via proximity and substrate shape complementarity. The approach generated a final enzyme variant with significantly increased turnover number and catalytic efficiency, demonstrating potential to overcome limitations in biocatalyst engineering and generate interesting building blocks.
ACS Catalysis 2023, 13, 5073-5083
For further information please contact:
Prof. Bernhard Hauer
Institute of Biochemistry and Technical Biochemistry,
Department Technical Biochemistry
University of Stuttgart
by Dongyang Chen, Francisco Tenopala-Carmona, Julius A. Knöller, Andreas Mischok, David Hall, Subeesh Madayanad Suresh, Tomas Matulaitis, Yoann Olivier, Pierre Nacke, Frank Gießelmann, Sabine Laschat, Malte C. Gather, Eli Zysman-Colman
The use of thermally activated delayed fluorescence (TADF) emitters and emitters that show preferential horizontal orientation of their transition dipole moment (TDM) are two emerging strategies to enhance the efficiency of OLEDs. We present the first example of a liquid crystalline multi-resonance TADF (MR-TADF) emitter, DiKTa-LC. The compound possesses a nematic liquid crystalline phase between 80 °C and 110 °C. Importantly, the TDM of the spin-coated film shows preferential horizontal orientation, with an anisotropy factor, a, of 0.28, which is preserved in doped poly(vinylcarbazole) films. Green-emitting (λEL = 492 nm) solution-processed OLEDs based on DiKTa-LC showed an EQEmax of 13.6%. We thus demonstrate for the first time how self-assembly of a liquid crystalline TADF emitter can lead to the so-far elusive control of the orientation of the transition dipole in solution-processed films, which will be of relevance for high-performance solution-processed OLEDs.
Angew. Chem. Int. Ed. 2023, e202218911.
For further information please contact
Prof. Sabine Laschat
Institute of Organic Chemistry
University of Stuttgart
Office of the Dean

Monika Carey
Secretary

Isabella Waldner
Dr.Faculty Manager