Epidermal Growth Factor Receptor (EGFR)
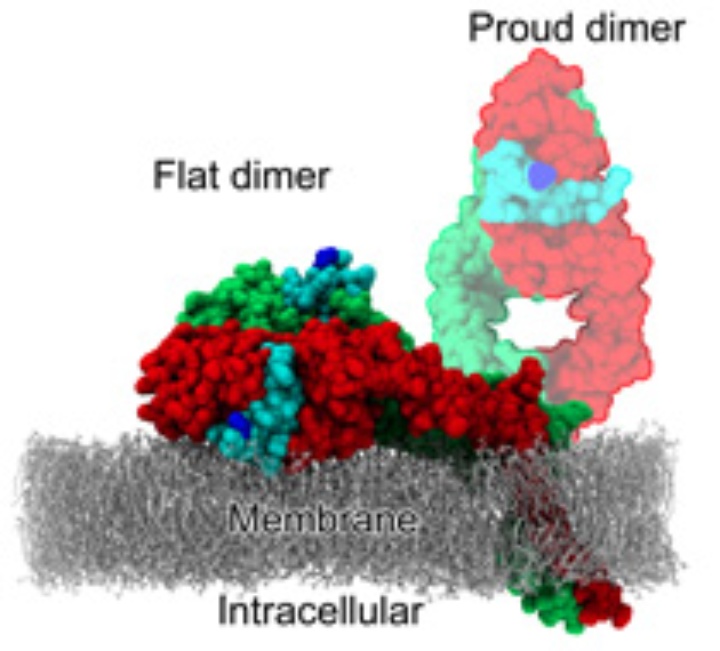
In humans, the epidermal growth factor receptor (EGFR, also called HER1, ErbB1) is one of a family of four cell receptors governing cell proliferation, differentiation, and death. The associated signaling network is implicated in the development of most human cancers and is one of the main targets of anti-cancer therapeutic agents. It is experimentally found in a high-affinity and a low-affinity form which differ in their geometry.
Single-molecule FRET experiments, performed at Daresbury and Rutherford Labs in the UK in the Lab-in-a-cell project, showed that in the high-affinity form, the ligands bound to the receptor come close to each other and to the membrane – both facts at variance with the models derived from crystal structures. The low-affinity form can well be explained by the crystal structures.
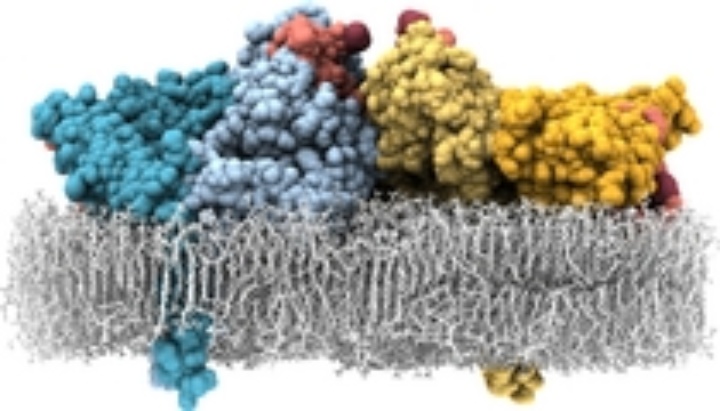
Using classical MD simulations we found [1,2] the receptor dimer with bound ligands being able to align flat on the membrane, explaining the low membrane–ligand distance. The alignment on the membrane, however, decreases the ligand binding affinity and, thus, does not explain the high-affinity form.
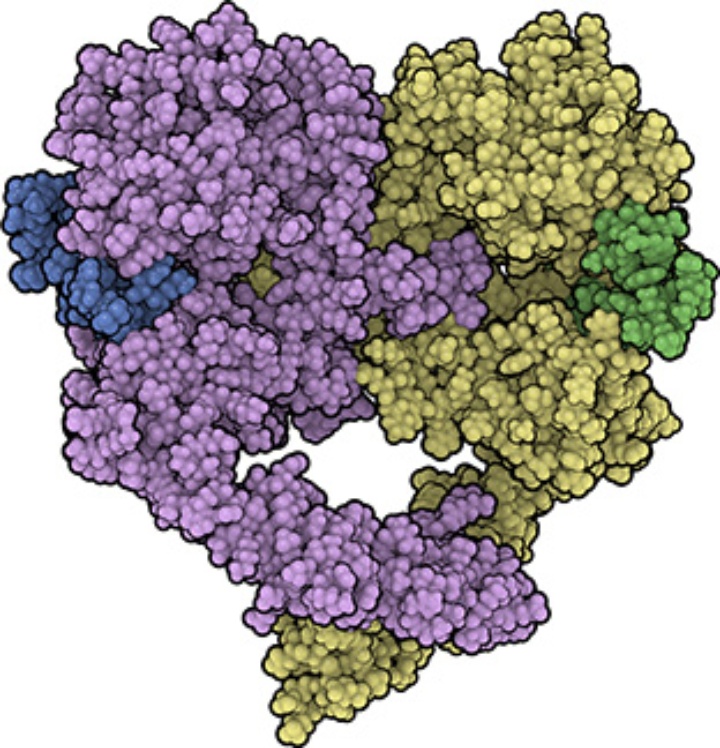
We also found an additional interface between two receptor-ligand complexes ("head-to-head interface") which is remarkably stable in MD simulations. This allows the formation of a tetramer of receptors (dimer of dimers, with four ligands bound). In this tetramer, the ligand-ligand distance is short, the ligands are close to the membrane and tightly bound to the receptors. Thus, it may serve as a consistent model for the high-affinity form of EGFR.
A light-weighted description of the EGFR system, our simulations and experiments can be found in Frontiers 2008.