Independant Work
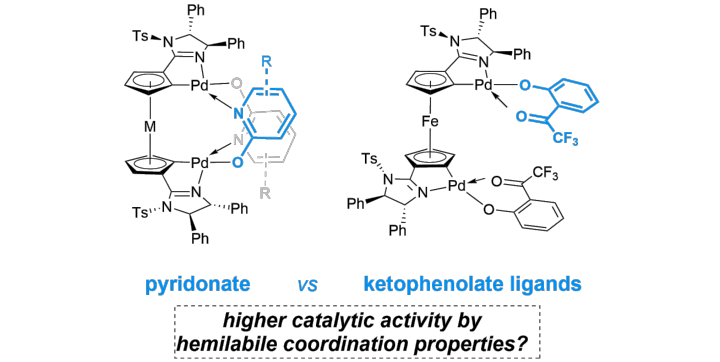
Planar Chiral Ferrocendiyl and Ruthenocendiyl Bisimidazoline Bispalladacycles Featuring Pyridin-2-olates and Ketophenolates as Potentially Hemilabile Ligands in Asymmetric 1,4-Additions
K. Dorst, W. Frey, G. Lu, R. Peters, Eur. J. Inorg.Chem. 2024, e202300748.
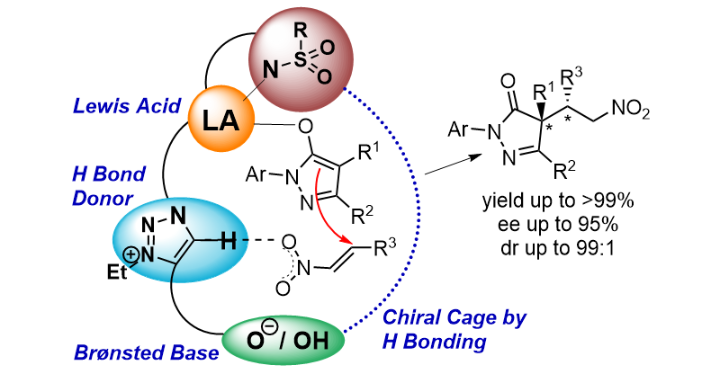
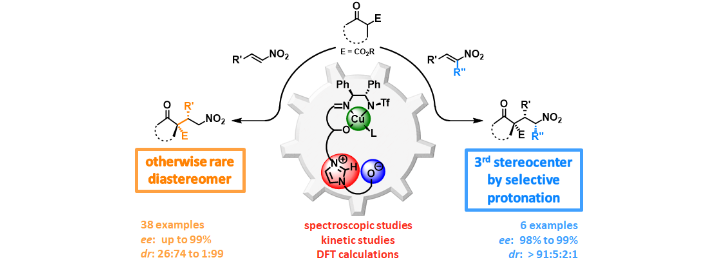
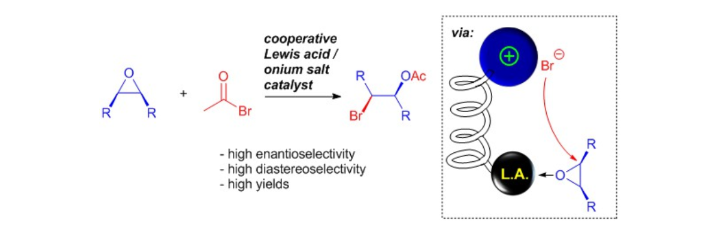
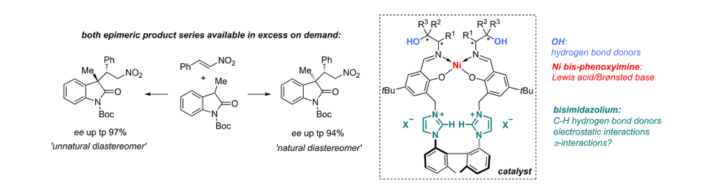
Cooperative Lewis Acid–1,2,3-Triazolium–Aryloxide Catalysis: Pyrazolone Addition to Nitroolefins as Entry to Diaminoamides
D. M. Wanner, P. M. Becker, S. Suhr, N. Wannenmacher, S. Ziegler, J. Herrmann, F. Willig, J. Gabler, K. Jangid, J. Schmid, A. C. Hans, W. Frey, B. Sarkar, J. Kästner, R. Peters, Angew. Chem. Int. Ed. 2023, 62, e202307317.
Kooperative Lewis-Säure-1,2,3-Triazolium-Aryloxid-Katalyse: Addition von Pyrazolonen an Nitroolefine als Zugang zu Diaminoamiden
D. M. Wanner, P. M. Becker, S. Suhr, N. Wannenmacher, S. Ziegler, J. Herrmann, F. Willig, J. Gabler, K. Jangid, J. Schmid, A. C. Hans, W. Frey, B. Sarkar, J. Kästner, R. Peters, Angew. Chem. 2023, 135, e202307317.
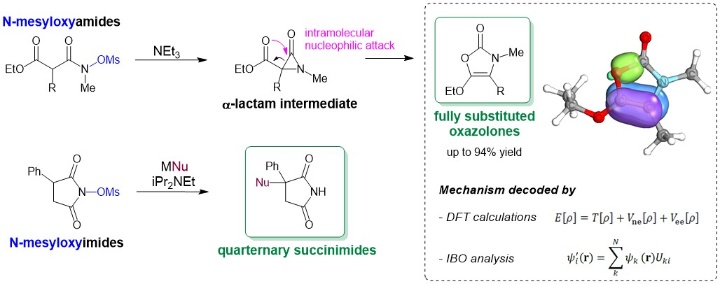
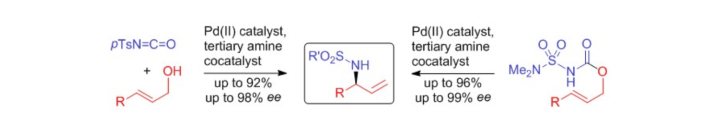
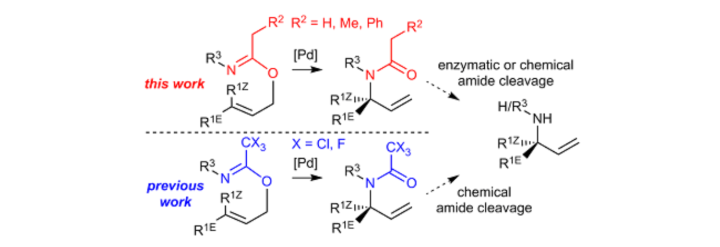
Use of the N–O Bonds in N-Mesyloxyamides and N-Mesyloxyimides To Gain Access to 5-Alkoxy-3,4-dialkyloxazol-2-ones and 3-Hetero-Substituted Succinimides: A Combined Experimental and Theoretical Study
L. Pfitzer, J. Heitkämper, J. Kästner, R. Peters, Synthesis 2023, 55, 2460-2472.
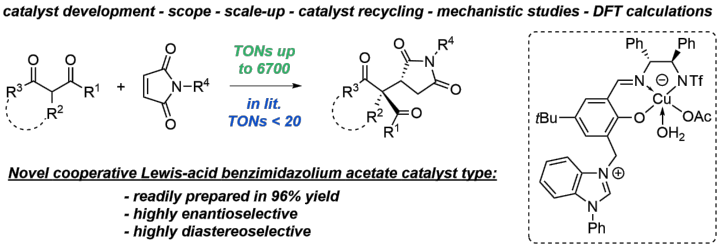
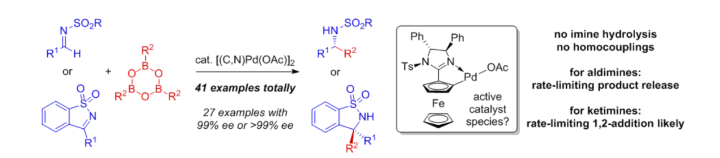
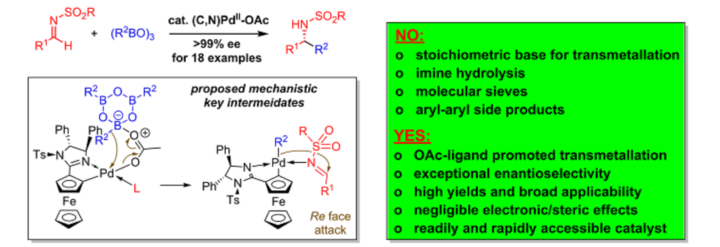
A Practical and Robust Zwitterionic Cooperative Lewis Acid / Acetate / Benzimidazolium Catalyst for Direct 1,4-Additions
A. C. Hans, P. M. Becker, J. Haußmann, S. Suhr, D. M. Wanner, V. Lederer, F. Willig, W. Frey, B. Sarkar, J. Kästner, R. Peters, Angew. Chem. Int. Ed. 2023, 62, e202217519.
Ein praktikabler und robuster zwitterionischer kooperativer Lewis-Säure-/Acetat-/Benzimidazolium-Katalysator für direkte 1,4-Additionen
A. C. Hans, P. M. Becker, J. Haußmann, S. Suhr, D. M. Wanner, V. Lederer, F. Willig, W. Frey, B. Sarkar, J. Kästner, R. Peters, Angew. Chem. 2023, 135, e202217519.
Stereoretentive Regio- and Enantioselective Allylation of Isoxazolinones by a Planar Chiral Palladacycle Catalyst
X. Yu, L. Hu, W. Frey, G. Lu, R. Peters, Angew. Chem. Int. Ed. 2022, 61, e202210145.
Stereoretentive regio- und enantioselektive Allylierung von Isoxazolinonen per planar chiralem Palladacyclus-Katalysator
X. Yu, L. Hu, W. Frey, G. Lu, R. Peters, Angew. Chem. 2022,134, e202210145.
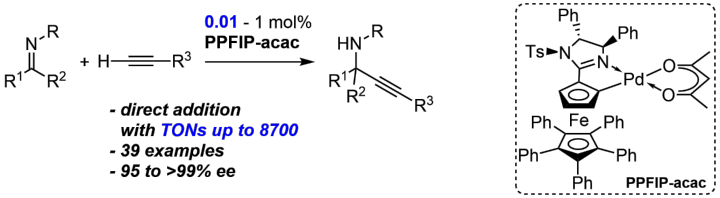
Direct Enantioselective Addition of Alkynes to Imines by a Highly Efficient Palladacycle Catalyst
C. Pfeffer, P. Probst, N. Wannenmacher, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2022, 61, e202206835.
Direkte enantioselektive Addition von Alkinen an Imine unter Verwendung eines hocheffizienten Palladacyclus als Katalysator
C. Pfeffer, P. Probst, N. Wannenmacher, W. Frey, R. Peters, Angew. Chem. 2022, 134, e202206835.
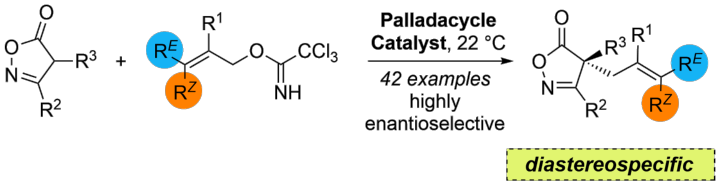
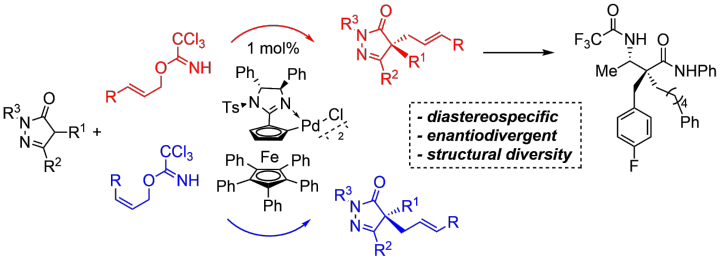
Diastereospecific Enantiodivergent Allylation of Pyrazolones as an Entry to β-Aminoamides
N. Wannenmacher, M. Heberle, X. Yu, A. Demircan, D. M. Wanner, C. Pfeffer, R. Peters, Adv. Synth. Catal. 2022, 364, 3396–3403.
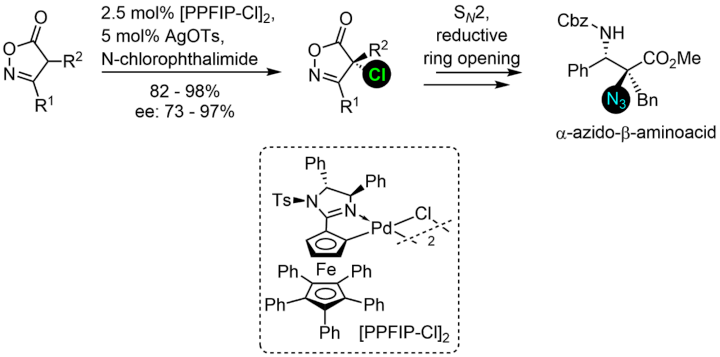
Catalytic Asymmetric Chlorination of Isoxazolinones
N. Wannenmacher, N. Keim, W. Frey, R. Peters, Eur. J. Org. Chem. 2022, e202200030.
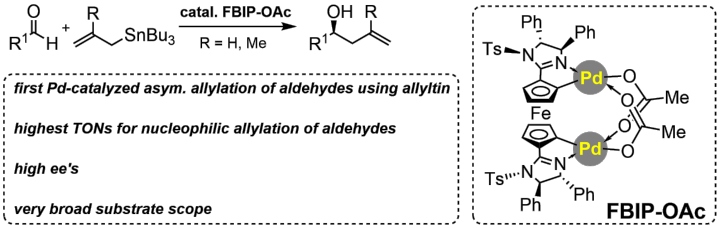
Bispalladacycle Catalyzed Nucleophilic Enantioselective Allylation of Aldehydes by Allylstannanes
M. Heberle, S. Legendre, N. Wannenmacher, M. Weber, W. Frey, R. Peters, ChemCatChem 2022, 14, e202200093.
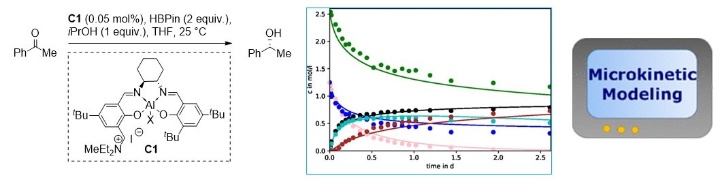
Asymmetric Hydroboration of Ketones by Cooperative Lewis Acid–Onium Salt Catalysis: A Quantum Chemical and Microkinetic Study to Combine Theory and Experiment.
J. Heitkämper, J. Herrmann, M. Titze, S. M. Bauch, R. Peters, J. Kästner, ACS Catal. 2022, 12, 1497–1507.
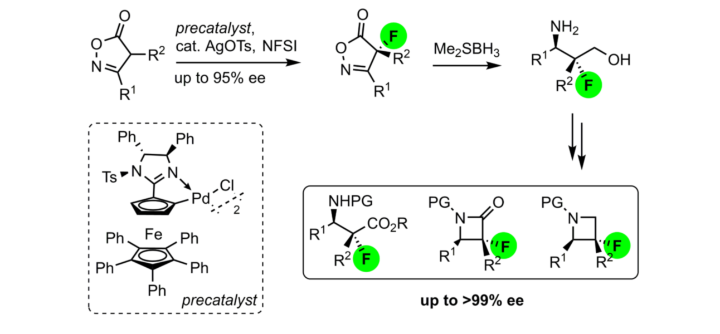
Enantioenriched γ-Aminoalcohols, β-Aminoacids, β-Lactams and Azetidines Featuring Tetrasubstituted Fluorinated Stereocenters via Palladacycle Catalyzed Asymmetric Fluorination of Isoxazolinones.
N. Wannenmacher, C. Pfeffer, W. Frey, R. Peters, J. Org. Chem. 2022, 87, 670–682.
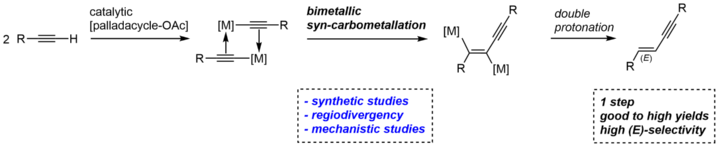
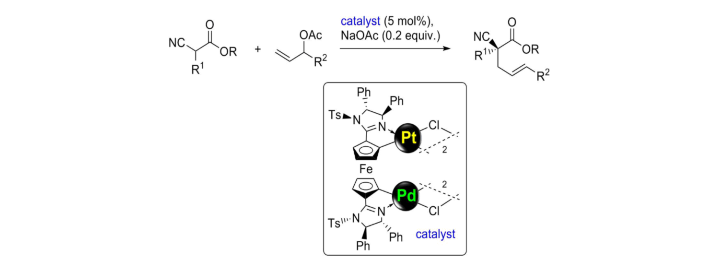
Stereo- and Regioselective Dimerization of Alkynes to Enynes by Bimetallic Syn-Carbopalladation.
C. Pfeffer, N. Wannenmacher, W. Frey, R. Peters, ACS Catal. 2021, 11, 5496–5505.
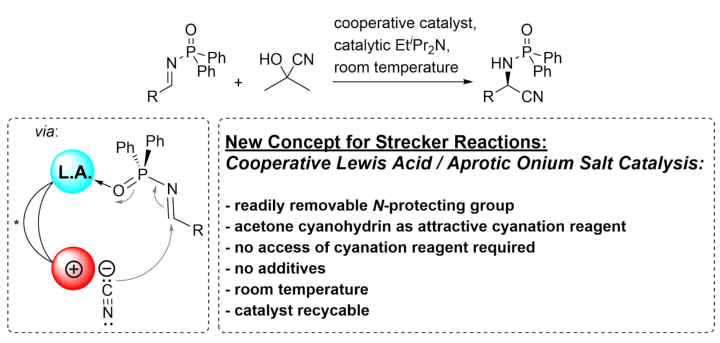
Asymmetric Hydrocyanation of N‐Phosphinoyl Aldimines with Acetone Cyanohydrin by Cooperative Lewis Acid / Onium Salt / Brønsted Base Catalysis.
T. Junge, M. Titze, W. Frey, R. Peters, ChemCatChem 2021,13, 1509-1512.
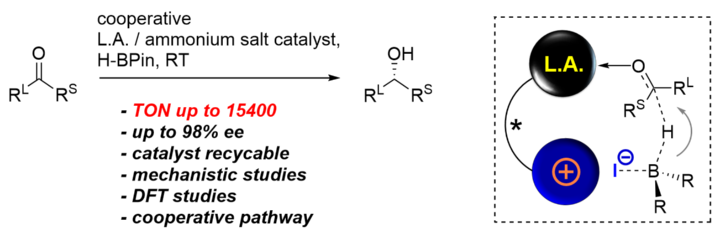
Highly Active Cooperative Lewis Acid – Ammonium Salt Catalyst for the Enantioselective Hydroboration of Ketones.
M. Titze, J. Heitkämper, T. Junge, J. Kästner, R. Peters, Angew. Chem. Int. Ed. 2021, 60, 5544-5553.
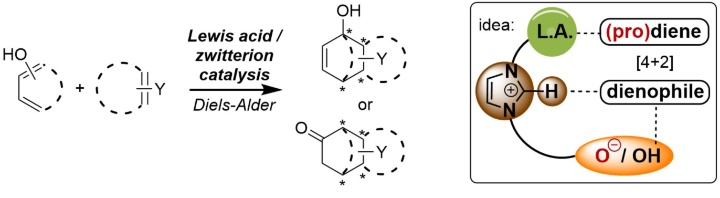
Enantiodivergent [4+2] Cycloaddition of Dienolates by Polyfunctional Lewis Acid/Zwitterion Catalysis.
V. Miskov-Pajic, F. Willig, D. M. Wanner, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2020, 59, 19873-19877.
Enantiodivergent [4+2] Cycloaddition of Dienolates by Polyfunctional Lewis Acid/Zwitterion Catalysis.
V. Miskov-Pajic, F. Willig, D. M. Wanner, W. Frey, R. Peters, Angew. Chem. 2020, 132, 20045-20049.
Stereospecific Asymmetric Synthesis of Tertiary Allylic Alcohol Derivatives by Catalytic [2,3]-Meisenheimer Rearrangements.
X. Yu, N. Wannenmacher, R. Peters, Angew. Chem. Int. Ed. 2020, 59, 10944-10948.
Stereospezifische asymmetrische Synthese tertiärer Allylalkohol‐ Derivate über katalytische [2,3]‐Meisenheimer‐Umlagerungen.
X. Yu, N. Wannenmacher, R. Peters, Angew. Chem. 2020, 132, 11037-11041.
Polyfunctional Imidazolium Aryloxide Betaine / Lewis Acid Catalysts as Tool for the Asymmetric Synthesis of Disfavored Diastereomers.
F. Willig, J. Lang, A. C. Hans, M. R. Ringenberg, D. Pfeffer, W. Frey, R. Peters, J. Am. Chem. Soc. 2019, 141, 12029-12043.
Correction
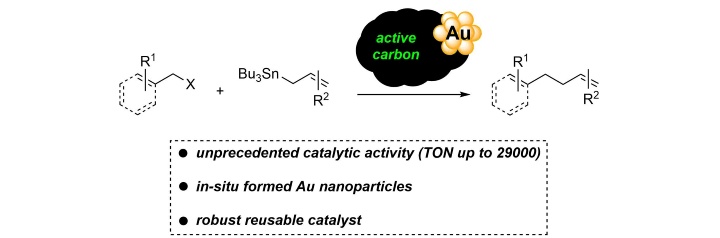
In-situ Generated Gold Nanoparticles on Active Carbon as Reusable Highly Efficient Catalysts for a Csp3–Csp3 Stille Coupling.
J. Holz, C. Pfeffer, H. Zuo, D. Beierlein, G. Richter, E. Klemm, R. Peters, Angew. Chem. Int. Ed. 2019, 58, 10330-10334.
In situ erzeugte Goldnanopartikel auf Aktivkohle als wiederverwendbare hocheffiziente Katalysatoren für eine Csp3-Csp3 Stille-Kupplung.
J. Holz, C. Pfeffer, H. Zuo, D. Beierlein, G. Richter, E. Klemm, R. Peters, Angew. Chem. 2019, 131, 10437-10442.
Highlighted by Nature Catalysis
Nature Catalysis, 2019, 2, 376.
SYNFACTS of the month in October 2019
Synfacts, 2019, 15, 1163.
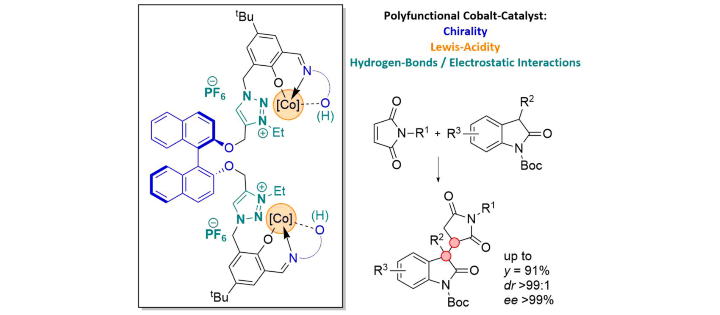
Polyfunctional Bis-Lewis-Acid-/Bis-Triazolium Catalysts for Stereoselective 1,4-Additions of 2-Oxindoles to Maleimides.
J. Schmid, T. Junge, J. Lang, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2019, 58, 5447-5451.
Polyfunktionelle Bis-Lewis-Säure-/Bis-Triazolium-Katalysatoren zur stereoselektiven 1,4-Addition von 2-Oxindolen an Maleimide.
J. Schmid, T. Junge, J. Lang, W. Frey, R. Peters, Angew. Chem. 2019, 131, 5501-5505.
Asymmetric Carboxycyanation of Aldehydes by Cooperative AlF‐/Onium Salt Catalysts: from Cyanoformate to KCN as Cyanide Source.
D. Brodbeck, S. Álvarez-Barcia, J. Meisner, F. Broghammer, J. Klepp, D. Garnier, W. Frey, J. Kästner, R. Peters, Chem. Eur. J. 2019, 25, 1515-1524.
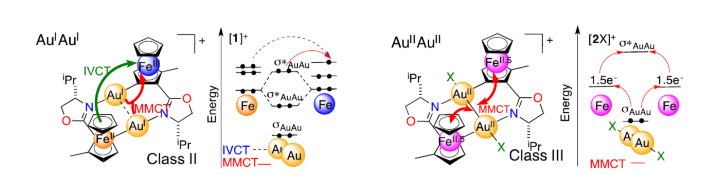
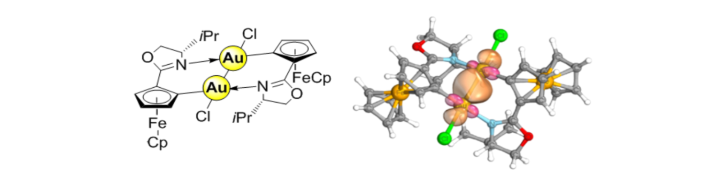
Intervalence of two planar chiral 2-methylferrocenyl groups over a diaurum bridge.
M. R. Ringenberg, J. Holz, R. Peters, Dalton Trans. 2018, 47, 12873-12878.
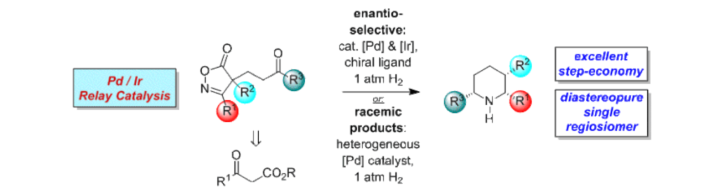
Regio-, Diastereo- and Enantioselective Synthesis of Piperidines with Three Stereogenic Centers from Isoxazolinones by Palladium / Iridium Relay Catalysis.
S. Rieckhoff, W. Frey, R. Peters, Eur. J. Org. Chem. 2018, 1797-1805.
Diastereoselective synthesis, structure and reactivity studies of ferrocenyloxazoline gold(I) and gold(II) complexes.
J. Holz, M. Ayerbe-García, W. Frey, F. Krupp, R. Peters, Dalton Trans. 2018, 47, 3880-3905.
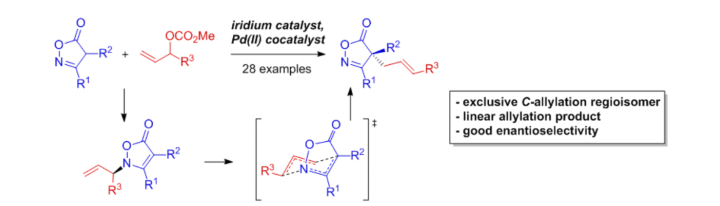
Double Regioselective Asymmetric C-Allylation of Isoxazolinones: Iridium Catalyzed N-Allylation Followed by an Aza-Cope-Rearrangement.
S. Rieckhoff, J. Meisner, J. Kästner, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2018, 57, 1404–1408.
Doppelt regioselektive asymmetrische C-Allylierung von Isoxazolinonen: Iridium-katalysierte N-Allylierung mit nachfolgender Aza-Cope-Umlagerung.
S. Rieckhoff, J. Meisner, J. Kästner, W. Frey, R. Peters, Angew. Chem. 2018, 130, 1418–1422.
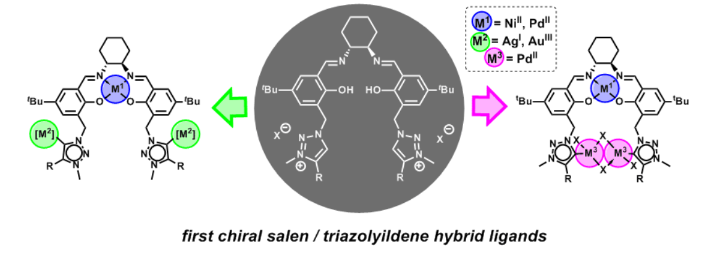
Polynuclear Enantiopure Salen–Mesoionic Carbene Hybrid Complexes.
J. Schmid, W. Frey, R. Peters, Organometallics 2017, 36, 4313–4324.
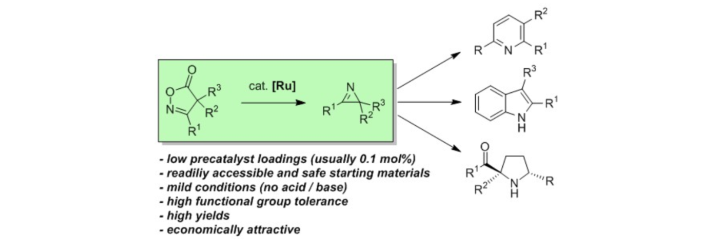
Ruthenium-Catalyzed Synthesis of 2H-Azirines from Isoxazolinones.
S. Rieckhoff, M. Titze, W. Frey, R. Peters, Org. Lett. 2017, 19, 4436–4439.
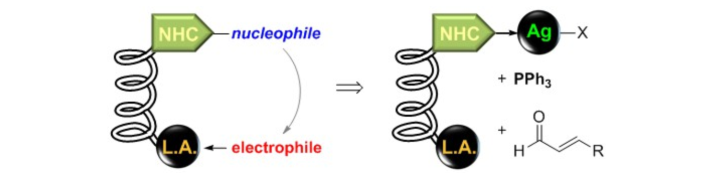
Titanium Salen Complexes with Appended Silver NHC Groups as Nucleophilic Carbene Reservoir for Cooperative Asymmetric Lewis Acid / NHC Catalysis.
K. Latendorf, M. Mechler, I. Schamne, D. Mack, W. Frey, R. Peters, Eur. J. Org. Chem. 2017, 28, 4140–4167.
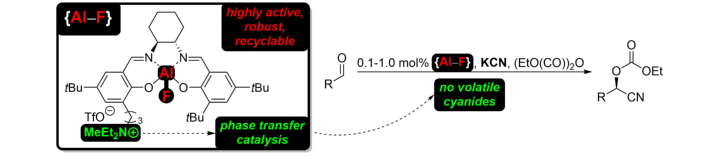
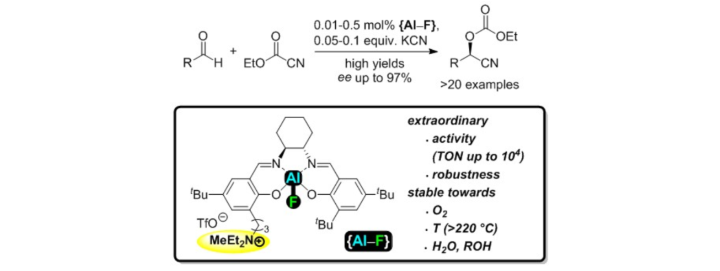
An Aluminum Fluoride Complex with an Appended Ammonium Salt as an Exceptionally Active Cooperative Catalyst for the Asymmetric Carboxycyanation of Aldehydes.
D. Brodbeck, F. Broghammer, J. Meisner, J. Klepp, D. Garnier, W. Frey, J.Kästner, R. Peters, Angew. Chem. Int. Ed. 2017, 56, 4056–4060.
Ein Aluminium-Fluorid-Komplex mit gekoppelter Ammonium-Einheit als außergewöhnlich aktiver kooperativer Katalysator in der asymmetrischen Carboxycyanierung von Aldehyden.
D. Brodbeck, F. Broghammer, J. Meisner, J. Klepp, D. Garnier, W. Frey, J.Kästner, R. Peters, Angew. Chem. 2017, 129, 4115–4119.
Cooperative Lewis acid–onium salt catalysis as tool for the desymmetrization of meso-epoxides.
F. Broghammer, D. Brodbeck, T. Junge, R. Peters, Chem. Commun. 2017, 53, 1156-1159.
open access
Highly Enantioselective Ferrocenyl Palladacycle-Acetate Catalysed Arylation of Aldimines and Ketimines with Arylboroxines.
C. Schrapel, W. Frey, D. Garnier, R. Peters, Chem. Eur. J. 2017, 23, 2448–2460.
Dual Palladium(II)/Tertiary Amine Catalysis for Asymmetric Regioselective Rearrangements of Allylic Carbamates.
J. M. Bauer, W. Frey, R. Peters, Chem. Eur. J. 2016, 22, 5767–5777.
Regioselective Asymmetric Allylic Alkylation Reaction of -Cyanoacetates Catalyzed by a Heterobimetallic Platina-/Palladacycle.
M. Weiss, J. Holz, R. Peters, Eur. J. Org. Chem. 2016, 210-227.
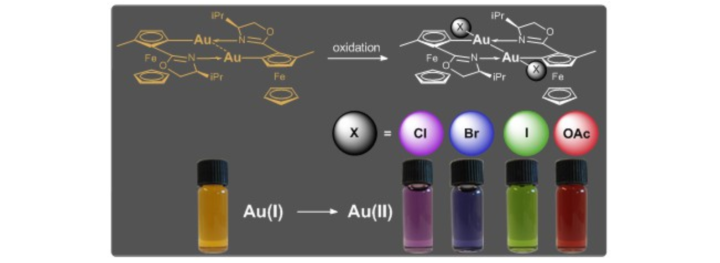
Dinuclear planar chiral ferrocenyl gold(I) & gold(II) complexes.
M. Ayerbe Garcia, W. Frey, M. Ringenberg, M. Schwilk, R. Peters, Chem.Commun. 2015, 51, 16806-16809.
open access
Diastereodivergent Asymmetric 1,4-Addition of Oxindoles to Nitroolefins by Using Polyfunctional Nickel-Hydrogen-Bond-Azolium Catalysts.
M. Mechler, R. Peters, Angew. Chem. Int. Ed. 2015, 54, 10303–10307.
Diastereodivergente asymmetrische 1,4-Additionen von Oxindolen an Nitroolefine durch polyfunktionelle Nickel-H-Brücken-Azolium-Katalysatoren.
M. Mechler, R. Peters, Angew. Chem. 2015, 127, 10442–10446.
Exogenous-Base-Free Palladacycle-Catalyzed Highly Enantioselective Arylation of Imines with Arylboroxines.
C. Schrapel, R. Peters, Angew. Chem. Int. Ed. 2015, 54, 10289–10293.
Palladacyclus-katalysierte, hoch enantioselektive Arylierung von Iminen mit Arylboroxinen ohne Verwendung einer exogenen Base.
C. Schrapel, R. Peters, Angew. Chem. 2015, 127, 10428–10432.
Regioselective Pd-Catalyzed Synthesis of 2,3,6-Trisubstituted Pyridines from Isoxazolinones.
S. Rieckhoff, T. Hellmuth, R. Peters, J. Org. Chem. 2015, 80, 6822–6830.
Catalytic asymmetric [3,3]-rearrangements of allylic acetimidates.
J. M. Bauer, R. Peters, Catal. Sci. Technol. 2015, 5, 2340-2346.
open access
Regioselective Catalytic Asymmetric C-Alkylation of Isoxazolinones by a Base-Free Palladacycle-Catalyzed Direct 1,4-Addition.
T. Hellmuth, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2015, 54, 2788–2791.
Regioselektive katalytische asymmetrische C-Alkylierung von Isoxazolinonen durch basenfreie Palladacyclus-katalysierte direkte 1,4-Addition.
T. Hellmuth, W. Frey, R. Peters, Angew. Chem. 2015, 127, 2829–2833.
Catalytic Direct Dehydrogenative Cross-Couplings of C−H (Pro)Nucleophiles and Allylic Alcohols without an Additional Oxidant.
M. Weiss, R. Peters, ACS Catal. 2015, 5, 310-316.
Bimetallic Catalysis: Cooperation of Carbophilic Metal Centers.
M. Weiss, R. Peters, in: Cooperative Catalysis – Designing Efficient Catalysts for Synthesis, R. Peters (Ed.), Wiley-VCH, Weinheim, 2015.
Cooperative Catalysis – Designing Efficient Catalysts for Synthesis.
R. Peters (Ed.), Wiley-VCH, Weinheim, 2015.
Heterogenization of ferrocene palladacycle catalysts on ROMP-derived monolithic supports and application to a Michael addition.
M. Sudheendran, S. H. Eitel, S. Naumann, M. R. Buchmeiser, R. Peters, New J. Chem. 2014, 38, 5597-5607.
Macrocyclic Salen−Bis-NHC Hybrid Ligands and Their Application to the Synthesis of Enantiopure Bi- and Trimetallic Complexes.
M. Mechler, W. Frey, R. Peters, Organometallics 2014, 33, 5492-5508.
Asymmetric Cascade Reaction to Allylic Sulfonamides from Allylic Alcohols by Palladium(II)/Base-Catalyzed Rearrangement of Allylic Carbamates.
J. M. Bauer, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2014, 53, 7634-7638.
Asymmetrische Kaskadenreaktion zu Allylsulfonamiden aus Allylalkoholen über eine Palladium(II)/Base-katalysierte Umlagerung von Allylcarbamaten.
J. M. Bauer, W. Frey, R. Peters, Angew. Chem. 2014, 126, 7764-7768.
Cooperative Bimetallic Asymmetric Catalysis: Comparison of a Planar Chiral Ruthenocene Bis-Palladacycle to the Corresponding Ferrocene.
T. Hellmuth, S. Rieckhoff, M. Weiss, K. Dorst, W. Frey, R. Peters, ACS Catal. 2014, 4, 1850-1858.
Author Profile René Peters.
R. Peters, Angew. Chem. Int. Ed. 2014, 53, 5499.
Autorenprofil René Peters.
R. Peters, Angew. Chem. 2014, 126, 5604.
Sterically Demanding Planar Chiral P,N Ligands by Diastereoselective Ortho Lithiation of Pentaphenylferrocenyloxazolines and Their Application to Palladium-Catalyzed Substitutions with Cyclic Allylic Acetates.
M. Ayerbe Garcia, W. Frey, R. Peters, Organometallics 2014, 33, 1068-1078.
Asymmetric Pd(II)-Catalyzed Cascade Reaction Giving Quaternary Amino Succinimides via 1,4-Addition and a Nef-Type-Reaction.
M. Weber, W. Frey, R. Peters, Angew. Chem. Int. Ed. 2013, 52, 13223-13227.
Asymmetrische Palladium(II)-katalysierte Kaskadenreaktion zu quartären Aminosuccinimiden über 1,4-Addition und eine Nef-artige Reaktion.
M. Weber, W. Frey, R. Peters, Angew. Chem. 2013, 125, 13465-13469.
Monomeric Ferrocene Bis-Imidazoline Bis-Palladacycles: Variation of Pd–Pd Distances by an Interplay of Metallophilic, Dispersive, and Coulombic Interactions.
M. Weber, J. E. M. N. Klein, B. Miehlich, W. Frey, R. Peters, Organometallics 2013, 32, 5810-5817.
Catalytic Asymmetric Synthesis of Spirocyclic Azlactones by a Double Michael Addition Approach.
M. Weber, W. Frey, R. Peters, Chem. Eur. J. 2013, 19, 8342-8351.
Asymmetric Michael Additions of α-Cyanoacetates by Soft Lewis Acid / Hard Brønsted Acid Catalysis: Stereodivergency With Bi- vs Monometallic Catalysts.
S. H. Eitel, S. Jautze, W. Frey, R. Peters, Chem. Sci. 2013, 4, 2218-2233.
For reprints please contact: rene.peters@oc.uni-stuttgart.de
Homo- and Hetero-Bimetallic Pd-, Ag- and Ni-Hybrid Salen―Bis-NHC Complexes.
M. Mechler, K. Latendorf, W. Frey, R. Peters, Organometallics 2013, 32, 112-130.
Pd(II)-Catalyzed Regio-, Enantio- and Diastereoselective 1,4-Addition of Azlactones Formed In-Situ From Racemic Unprotected Amino Acids and Acetic Anhydride.
M. Weber, R. Peters, J. Org. Chem. 2012, 77, 10846–10855.
Asymmetric Synthesis of Hetero-Bimetallic Planar Chiral Ferrocene Pallada-/Platinacycles and their Application to Enantioselective Aza-Claisen Rearrangements.
M. Weiss, W. Frey, R. Peters, Organometallics 2012, 31, 6365–6372.
Bispalladacycle Catalyzed Michael Addition of In-Situ Formed Azlactones to Enones.
M. Weber, S. Jautze, W. Frey, R. Peters Chem. Eur. J. 2012, 18, 14792–14804.
Crystal structure of pentaphenylferrocenium tetrafluoroborate, C40H30BF4Fe.
W. Frey, S. H. Eitel, R. Peters, Z. Kristallogr. NCS 2012, 227, 559-561.
open access
Cooperative Al(Salen)-Pyridinium Catalysts for the Asymmetric Synthesis of trans-Configured β-Lactones by [2+2]-Cyclocondensation of Acylbromides and Aldehydes: Investigation of Pyridinium Substituent Effects.
P. Meier, F. Broghammer, K. Latendorf, G. Rauhut, R. Peters, Molecules 2012, 17, 7121–7150. (Special Issue Asymmetric Catalysis).
open access
Catalytic Asymmetric Synthesis of Functionalized α,α-Disubstituted α-Amino Acid Derivatives from Racemic Unprotected α-Amino Acids via In-Situ Generated Azlactones.
M. Weber, W. Frey, R. Peters, Adv. Synth. Catal. 2012, 354, 1443–1449.
Paramagnetic Palladacycles with PdIII Centers are Highly Active Catalysts for Asymmetric Aza-Claisen Rearrangements.
S. H. Eitel, M. Bauer, D. Schweinfurth, N. Deibel, B. Sarkar, H. Kelm, H.-J. Krüger, W. Frey, R. Peters, J. Am. Chem. Soc. 2012, 134, 4683–4693.
Isomerizations to Form a Stereogenic Center and Allylic Rearrangements.
S. Jautze, R. Peters, In Science of Synthesis: Stereoselective Synthesis, Evans, P. A.,
Ed.; Thieme: Stuttgart, 2011; Vol. 3, Chapter 3.10, pp 443–467.
Lewis Acid/Base Catalyzed [2+2]-Cycloaddition of Sulfenes and Aldehydes: A Versatile Entry to Chiral Sulfonyl and Sulfinyl Derivatives.
F. M. Koch, R. Peters, Chem. Eur. J. 2011, 17, 3679–3692.
Ferrocene and Half Sandwich Complexes as Catalysts with Iron Participation.
R. Peters, D. F. Fischer, S. Jautze, Top. Organomet. Chem.2011, 33, 139–175.
Bispalladacycle-Catalyzed Brønsted Acid/Base-Promoted Asymmetric Tandem Azlactone Formation−Michael Addition.
M. Weber, S. Jautze, W. Frey, R. Peters, J. Am. Chem. Soc. 2010, 132, 12222–12225.
Catalytic Asymmetric Synthesis of trans-Configured β-Lactones: Cooperation of Lewis Acid and Ion Pair Catalysis.
T. Kull, J. Cabrera, R. Peters, Chem. Eur. J. 2010, 16, 9132–9139.
Catalyst versus Substrate Induced Selectivity: Kinetic Resolution by Palladacycle Catalyzed Allylic Imidate Rearrangements.
R. Peters, Z.-q. Xin, F. Maier, Chem. Asian J. 2010, 5, 1770–1774.
Synthesis of Densely Substituted Trans-Configured 4-Acylated Piperidine-2,4-diones as 3:1 Adducts of Imines and Ketenes.
J. Cabrera, T. Hellmuth, R. Peters, J. Org. Chem. 2010, 75, 4326–4329.
Chiral Ferrocenes in Asymmetric Catalysis. Synthesis and Applications.(book review)
R. Peters, Angew. Chem. Int. Ed. 2010, 49, 4163–4164. Angew. Chem. 2010, 122, 4258.
Catalytic Asymmetric Formation of δ-Lactones from Unsaturated Acyl Halides.
P. S. Tiseni, R. Peters, Chem. Eur. J. 2010, 16, 2503–2517.
Catalytic Asymmetric Michael Additions of α-Cyano Acetates.
S. Jautze, R. Peters, Synthesis 2010, 365–388.
The Asymmetric Aza-Claisen Rearrangement: Development of Widely Applicable Pentaphenylferrocenyl Palladacycle Catalysts.
D. F. Fischer, A. Barakat, Z.-q. Xin, M. E. Weiss, R. Peters, Chem. Eur. J. 2009, 15, 8722–8741.
Catalytic Asymmetric Synthesis of β-Sultams as Precursors for Taurine Derivatives.
M. Zajac, R. Peters, Chem. Eur. J. 2009, 15, 8204–8222.
Diastereoselective Bis-Cyclopalladation of Ferrocene-1,1′-diyl Bis-Imidazolines: Translation of Central via Axial into Planar Chirality.
S. Jautze, S. Diethelm, W. Frey, R. Peters, Organometallics 2009, 28, 2001–2004.
Acid Catalysis in Modern Organic Synthesis, Vol. 1+2.(book review).
D. F. Fischer, R. Peters, Angew. Chem. Int. Ed. 2009, 48, 640; Angew. Chem. 2009, 121, 650.
A Highly Strained Planar Chiral Platinacycle for Catalytic Activation of Internal Olefins in the Friedel-Crafts Alkylation of Indoles.
H. Huang, R. Peters, Angew. Chem. Int. Ed. 2009, 48, 604–606.
Ein hoch-gespannter planar chiraler Platinacyclus zur katalytischen Aktivierung interner Olefine in Friedel-Crafts-Alkylierungen von Indolen.
Angew. Chem. 2009, 121, 612–615.
Bimetallic Enantioselective Catalysis of Michael Additions Forming Quaternary Stereocenters.
S. Jautze, R. Peters, Angew. Chem. Int. Ed. 2008, 47, 9284–9288;
Enantioselektive Dimetallkatalyse von Michael-Additionen zur Bildung quartärer Stereozentren.
Angew. Chem. 2008, 120, 9424–9429.
Catalytic Methods for Direct Access to Chiral High-Added-Value Products.(account)
R. Peters, D. F. Fischer, S. Jautze, F. M. Koch, T. Kull, P. S. Tiseni, Z.-q. Xin, M. Zajac, Chimia 2008, 62, 497–505.
Contact Ion Pair (CIP) Directed Lewis-Acid Catalysis: Asymmetric Formation of trans-Configured β-Lactones.
T. Kull, R. Peters, Angew. Chem. Int. Ed. 2008, 47, 5461–5464;
Kontaktionenpaar-gesteuerte Lewis-Säurekatalyse zur asymmetrischen Synthese von trans-konfigurierten β-Lactonen.
Angew. Chem. 2008, 120, 5541-5544.
Rapid Asymmetric Access to β-Hydroxysulfinic Acids and Allylsulfonic Acids by Chemoselective Reduction of β-Sultones.
F. M. Koch, R. Peters, Synlett 2008, 1505–1509.
Catalytic Asymmetric Formation of Secondary Allylic Amines by Aza-Claisen Rearrangement of Trifluoroacetimidates.
Z.-q. Xin, D. F. Fischer, R. Peters, Synlett 2008, 1495–1499.
Lewis Acid-Lewis Base Catalyzed Enantioselective Hetero-Diels-Alder Reaction for Direct Access to δ-Lactones.
P. S. Tiseni, R. Peters, Org. Lett. 2008, 10, 2019–2022.
Synthesis of nearly Enantiopure Allylic Amines by Aza-Claisen Rearrangement of Z-Configured Allylic Trifluoroacetimidates Catalyzed by Highly Active Ferrocenylbispalladacycles.
S. Jautze, P. Seiler, R. Peters, Chem. Eur. J. 2008, 14, 1430–1444.
Asymmetric Formation of Allylic Amines with N-Substituted Quaternary Stereocenters by Pd(II)-Catalyzed Aza-Claisen Rearrangements.
D. F. Fischer, Z.-q. Xin, R. Peters, Angew. Chem. Int. Ed. 2007, 46, 7704-7707;
Asymmetrische Synthese von Allylaminen mit N-substituierten quartären Stereozentren durch Palladium(II)-katalysierte Aza-Claisen-Umlagerungen.
Angew. Chem. 2007, 119, 7848–7851.
Practical Enantioselective Synthesis of β-Lactones Catalyzed by Aluminum Bissulfonamide Complexes.
T. Kull, R. Peters, Adv. Synth. Catal. 2007, 349, 1647–1652.
Catalytic Asymmetric Formation of δ-Lactones by [4+2]-Cycloaddition of Zwitterionic Dienolates Generated from α,β-Unsaturated Acid Chlorides.
P. S. Tiseni, R. Peters, Angew. Chem. Int. Ed. 2007, 46, 5325-5328;
Katalytische asymmetrische Synthese von δ-Lactonen durch [4+2]-Cycloaddition von zwitterionischen Dienolaten, erzeugt aus α,β-ungesättigten Säurechloriden.
Angew. Chem. 2007, 119, 5419–5422.
Catalytic Asymmetric Formation of β-Sultams.
M. Zajac, R. Peters, Org. Lett. 2007, 9, 2007–2010.
First Catalytic Enantio- and Diastereoselective Formation of β-Sultones: Ring-Strained Precursors for Enantioenriched β-Hydroxysulfonyl Derivatives.
F. M. Koch, R. Peters, Angew. Chem. Int. Ed. 2007, 46, 2685-2689;
Katalytische enantio- und diastereoselektive Synthese von β-Sultonen: ringgespannte Vorstufen für enantiomerenangereicherte β-Hydroxysulfonylderivate.
Angew. Chem. 2007, 119, 2739–2743.
Macrocyclic Ferrocenyl Bisimidazoline Palladacycle Dimers (FBIPs) as Highly Active and Enantioselective Catalysts for the Aza-Claisen Rearrangement of (Z)-Configured N-para-Methoxyphenyl Trifluoroacetimidates.
S. Jautze, P. Seiler, R. Peters, Angew. Chem. Int. Ed. 2007, 46, 1260–1264;
Makrocyclische Ferrocenyl-Bisimidazolin-Palladacyclus-Dimere als hoch aktive und enantioselektive Katalysatoren für die Aza-Claisen-Umlagerung von Z-konfigurierten N-para-Methoxyphenyltrifluoracetimidaten.
Angew. Chem. 2007, 119, 1282–1286.
Total Syntheses of the Antibacterial Natural Product Abyssomicin C. (highlight article)
R. Peters, D. F. Fischer, Angew. Chem. Int. Ed. 2006, 45, 5736–5739;
Totalsynthesen des antibakteriellen Naturstoffes Abyssomicin C. (highlight article)
Angew. Chem. 2006, 118, 5866–5869.
Practical, Highly Active and Enantioselective Ferrocenyl-Imidazoline Palladacycle Catalysts (FIPs) for the Aza-Claisen Rearrangement of N-para-Methoxyphenyl Trifluoroacetimidates.
M. E. Weiss, D. F. Fischer, Z.-q. Xin, S. Jautze, W. B. Schweizer, R. Peters, Angew. Chem. Int. Ed. 2006, 45, 5694–5699;
Praktikable, hochaktive und enantioselektive Ferrocenyl-Imidazolin-Palladacyclus(FIP)-Katalysatoren für die Aza-Claisen-Umlagerung von N-para-Methoxyphenyltrifluor-acetimidaten.
Angew. Chem. 2006, 118, 5823–5827.
Synthesis and Diastereoselective Ortho-Lithiation / Cyclopalladation of Enantiopure [2-Imidazolyl]-1’,2’,3’,4’,5’-pentamethylferrocenes and -1’,2’,3’,4’,5’-pentaphenylferrocenes.
R. Peters, Z.-q. Xin, D. F. Fischer, W. B. Schweizer, Organometallics 2006, 25, 2917–2920.
Preparation and Diastereoselective ortho-Metalation of Chiral Ferrocenyl Imidazolines: Remarkable Influence of LDA as Metalation Additive.
R. Peters, D. F. Fischer, Org. Lett. 2005, 7, 4137–4140.
F. Hoffmann-La Roche
Practical Racemic and Asymmetric Formal Total Syntheses of the Homocamptothecin Derivative and Anticancer Agent Diflomotecan via Tertiary Homoallylic Alcohols as Masked Aldol Equivalents.
R. Peters, C. Diolez, A. Rolland, E. Manginot, M. Veyrat, Heterocycles 2007, 72 (Special Issue dedicated to Prof. Yoshito Kishi on the occasion of his 70th birthday), 255-273.
Practical Formal Total Syntheses of the Homocamptothecin Derivative and Anti Cancer Agent Diflomotecan via Asymmetric Acetate Aldol Additions to Pyridine Ketone Substrates.
R. Peters, M. Althaus, C. Diolez, A. Rolland, E. Manginot, M. Veyrat, J. Org. Chem. 2006, 71, 5783-5795.
Process for the manufacture of intermediates in camptothecin production.
R. Peters, PCT Int. Appl. 2006, US 2006189807.
Novel processes for the production of 3-(pyridin-4-yl)-3-hydroxypentanoic acid intermediates.
C. Diolez, E. Manginot, R. Peters, A. Rolland, M. Veyrat, PCT Int. Appl. 2006, WO 2006033011.
Practical Formal Total Synthesis of (rac) and (S)-Camptothecin.
R. Peters, M. Althaus, A.-L. Nagy, Org. Biomol. Chem. 2006, 4, 498-509.
Efficient Synthesis of a 5-HT2C Receptor Agonist Precursor.
R. Peters, P. Waldmeier, A. Joncour, Org. Proc. Res. Develop. 2005, 9, 508-512.
Ph. D. and Postdoctoral Studies
Unified Total Synthesis of Pteriatoxins and Their Diastereomers.
F. Matsuura, R. Peters, M. Anada, S. S. Harried, J. Hao, Y. Kishi, J. Am. Chem. Soc. 2006, 128, 7463-7465.
The SAMP-/RAMP-hydrazone methodology in asymmetric synthesis. (review)
A. Job, C. Janeck, W. Bettray, R. Peters, D. Enders, Tetrahedron 2002, 58, 2253-2329.
Asymmetric Synthesis of Novel Ferrocenyl Ligands with Planar and Central Chirality and their Application to Pd-Catalyzed Allylic Substitution.
D. Enders, R. Peters, R. Lochtman, G. Raabe, J. Runsink, J. W. Bats, Eur. J. Org. Chem. 2000, 3399-3426.
Asymmetric Synthesis of Novel Ferrocenyl Ligands with Planar and Central Chirality and Their Application to Pd‐Catalyzed Allylic Substitutions.
D. Enders, R. Peters, R. Lochtman, J. Runsink, Eur. J. Org. Chem. 2000, 2839-2850.
Recovery of Carbonyl Compounds from N,N-Dialkylhydrazones. (account)
D. Enders, L. Wortmann, R. Peters, Acc. Chem. Res. 2000, 33, 157-169.
Novel Ferrocenyl Ligands with Planar and Central Chirality in Pd-Catalyzed Allylic Substitutions.
D. Enders, R. Peters, J. Runsink, J. W. Bats, Org. Lett. 1999, 1, 1863-1866.
Asymmetric Synthesis of Novel Ferrocenyl Ligands with Planar and Central Chirality.
D. Enders, R. Peters, R. Lochtman, G. Raabe, Angew. Chem. Int. Ed. 1999, 38, 2421-2423;
Asymmetrische Synthese neuer planar- und zentral-chiraler Ferrocenylliganden.
Angew. Chem. 1999, 111, 2579-2581.
Enantioselective Synthesis of Planar Chiral ortho-Functionalised Ferrocenylketones.
D. Enders, R. Peters, R. Lochtman, J. Runsink, Synlett 1997, 1462-1464.
Kontakt